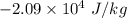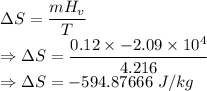Physics, 09.12.2019 18:31 starfox5454
What is the change in entropy of helium gas with total mass 0.120 kg at the normal boiling point of helium when it all condenses isothermally to liquid helium? assume that the normal boiling point of helium is 4.216 k and the heat of vaporization of helium is 2.09×10^4 j/kg .

Answers: 2
Another question on Physics

Physics, 21.06.2019 20:00
The first law of thermodynamics states that heat added to a system is neither created nor destroyed but is transformed ⇒ as it changes into other forms of energy.
Answers: 1


Physics, 22.06.2019 12:30
Consider a 1000 w iron whose base plate is made of 0.5 cm thick aluminum alloy 2024-t6 (ρ = 2770 kg/m3 and cp = 875 j/kg°c). the base plate has a surface area of 0.03 m2. initially, the iron is in thermal equilibrium with the ambient air at 22°c. assuming 90% of the heat generated in the resistance wires is transferred to the plate, determine the minimum time needed for the plate temperature to reach 200°c.
Answers: 1

Physics, 22.06.2019 13:40
Which of the following is not a transverse wave? a) soundb) lightc) radiod) all of thesee) none of these
Answers: 1
You know the right answer?
What is the change in entropy of helium gas with total mass 0.120 kg at the normal boiling point of...
Questions

Mathematics, 12.02.2020 04:19

Mathematics, 12.02.2020 04:19







Geography, 12.02.2020 04:19


Computers and Technology, 12.02.2020 04:19









Mathematics, 12.02.2020 04:19

 = Heat of vaporization =
= Heat of vaporization =