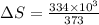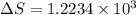Physics, 09.12.2019 20:31 cassiemyers60
Calculate the change in entropy when 1.00 kg of water at 100 degree c is vaporized and converted to steam at 100 degree c. assume that the heat of vaporization of water is 2256 times 103 j/kg. calculate the change in entropy when 1.00 kg of ice is melted at 0 degree c. assume that the heat of fusion of water is lf = 3.34 times 105j/kg. is the change entropy greater for melting or for vaporization? the change entropy greater for melting the change entropy greater for vaporization

Answers: 3
Another question on Physics

Physics, 21.06.2019 19:30
Ben(55 kg) is standing on very slippery ice when junior(25kg) bumps into him. junior was moving at a speed of 8m/s before the collision and ben and junior embrace after the collision. find the speed of ben and junior as they move across the ice after the collision .
Answers: 2

Physics, 21.06.2019 22:40
Which feature indicates that mars resides in the inner region of the solar system
Answers: 3

Physics, 22.06.2019 01:40
In all trials, the magnitude of the final velocity for g1 + g2 was less than the magnitude of any initial velocity. as mass increased, what happened to the velocity? the velocity decreased. the velocity increased. the velocity of g1 + g2 could not be measured. the velocity was not affected by the mass increase.
Answers: 1

Physics, 22.06.2019 04:30
Work out sian speed for the first 30 minutes of her journey. give your answer in km/h.
Answers: 1
You know the right answer?
Calculate the change in entropy when 1.00 kg of water at 100 degree c is vaporized and converted to...
Questions


Mathematics, 23.01.2021 19:40

Business, 23.01.2021 19:40




Mathematics, 23.01.2021 19:40



Mathematics, 23.01.2021 19:40

Mathematics, 23.01.2021 19:40

Mathematics, 23.01.2021 19:40

Mathematics, 23.01.2021 19:40


Mathematics, 23.01.2021 19:40

Mathematics, 23.01.2021 19:40




Mathematics, 23.01.2021 19:40

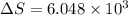
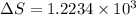
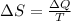
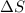 is change of entropy,
is change of entropy, 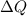 is change of heat and T is absolute temperature in kelvin
is change of heat and T is absolute temperature in kelvin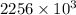 J/kg.
J/kg.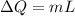
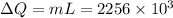
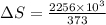
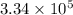 J/kg.
J/kg.![3.34 \times 10^{5}=334 \times 10^{3} J/kg.[tex]\Delta Q = mL = 334 \times 10^{3}](/tpl/images/0410/3794/17bda.png)