

Answers: 2
Another question on Physics

Physics, 21.06.2019 15:40
Translation can be considered to have three phases or steps: initiation, protein synthesis, and termination. give a detailed summary of the factors and molecules involved with each step and, where appropriate, compare/contrast the differences between prokaryotic and eukaryotic translation.
Answers: 1

Physics, 22.06.2019 02:00
The image shows a pendulum in simple harmonic motion the pendulum starts at a and swing to e
Answers: 3

Physics, 22.06.2019 05:30
Will give brainliest! which statement best describes the difference between strong nuclear forces and weak nuclear forces? weak nuclear forces are involved when certain types of atoms break down. strong nuclear forces are responsible for holding atoms' nucleus together. weak nuclear forces hold bonds between atoms together. strong nuclear forces hold together the nucleus of an atom. strong nuclear bonds prevent atoms from falling apart. weak nuclear bonds prevent compounds from falling apart. strong nuclear forces are involved in breaking electrons from their shells. weak nuclear forces hold protons in the nucleus.
Answers: 3

Physics, 22.06.2019 08:00
You have a pick-up truck that weighed 4,000 pounds when it was new. you are modifying it to increase its ground clearance. when you are finished
Answers: 1
You know the right answer?
Two vessels are labeled a and b. vessel a contains nh3 gas at 76°c, and vessel b contains ne gas at...
Questions

Mathematics, 07.10.2020 14:01

History, 07.10.2020 14:01

English, 07.10.2020 14:01




Mathematics, 07.10.2020 14:01


Mathematics, 07.10.2020 14:01

Chemistry, 07.10.2020 14:01



Mathematics, 07.10.2020 14:01


Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

English, 07.10.2020 14:01


History, 07.10.2020 14:01

![\pi _{rms}=656.77m/sExplanation: the following formula can be used for deriving the root mean square velocity[tex]\pi _{rms} =\sqrt({3RT)/M}](/tpl/images/0410/9487/b3152.png)
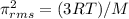
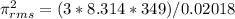
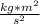 /(kg)
/(kg)

