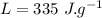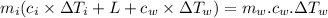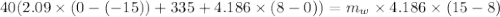A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) containing water at 15.00°c.
when equilibrium is reached, the final temperature is 8.00°c.
how much water did the calorimeter contain initially?
the specific heat of ice is 2090 j/kg • k, that of water is 4186 j/kg • k, and the latent heat of fusion of water is 33.5 × 104 j/kg.

Answers: 1
Another question on Physics

Physics, 22.06.2019 05:00
Aperson walking 1 mile everyday for exercise, leaving her front porch at 9: 00 am and returning to her front porch at 9: 25 am. what is the total displacement of her daily walk? a) 1 mileb) 0c) 25 minutes d) none of the above
Answers: 1

Physics, 22.06.2019 07:00
Suppose while hauling rocks, you accidentally drop one. it breaks apart in flat, planar sections. what type of rock did you just drop? 20 points
Answers: 3

Physics, 22.06.2019 09:10
The air that we breath is made mostly of which gaseous molecule
Answers: 1

Physics, 22.06.2019 12:00
The sun’s mass is 2.0×10^ 30 kg, its radius is 7.0×10 5 km, and it has a rotational period of approximately 28 days. if the sun should collapse into a white dwarf of radius 3.5×10 3 km, what would its period be if no mass were ejected and a sphere of uniform density can model the sun both before and after?
Answers: 3
You know the right answer?
A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) contain...
Questions





Physics, 31.08.2019 22:30


History, 31.08.2019 22:30


Mathematics, 31.08.2019 22:30

World Languages, 31.08.2019 22:30

Spanish, 31.08.2019 22:30

Social Studies, 31.08.2019 22:30



Social Studies, 31.08.2019 22:30


Biology, 31.08.2019 22:30

Health, 31.08.2019 22:30


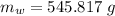
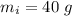 initial temperature of ice block,
initial temperature of ice block,  initial temperature of water,
initial temperature of water,  final temperature of mixture,
final temperature of mixture, 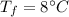 specific heat of ice,
specific heat of ice, 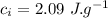 specific heat of water,
specific heat of water, 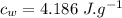 Latent heat of fusion of water,
Latent heat of fusion of water,