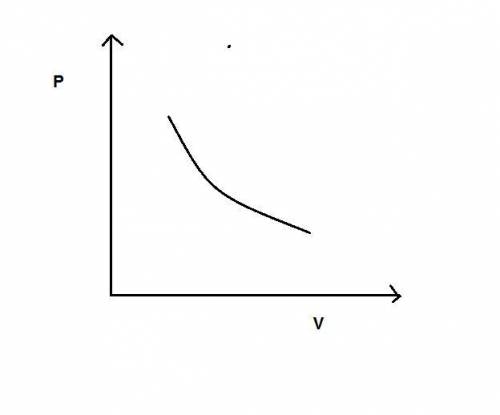
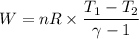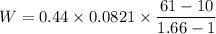During an adiabatic expansion the temperature of 0.440 mol of argon (ar) drops from 61.0 ∘c to 10.0 ∘c. the argon may be treated as an ideal gas.(a) draw a pv-diagram for this process.
(b) how much work does the gas do?
(c) what is the change in internal energy of the gas? explain.

Answers: 1
Another question on Physics

Physics, 21.06.2019 20:50
Identify the type of synthetic material used in each object
Answers: 1


Physics, 22.06.2019 15:50
Decreased sensitivity to an unchanging stimulus is known as
Answers: 3

Physics, 22.06.2019 19:40
Two charged particles, q1 and q2, are located on the x-axis, with q1 at the origin and q2 initially at x1 = 12.2 mm. in this configuration, q1 exerts a repulsive force of 2.62 µn on q2. particle q2 is then moved to x2 = 18.0 mm. what is the force (magnitude and direction) that q2 exerts on q1 at this new location? (give the magnitude in µn.)
Answers: 1
You know the right answer?
During an adiabatic expansion the temperature of 0.440 mol of argon (ar) drops from 61.0 ∘c to 10.0...
Questions

Mathematics, 20.09.2019 20:30


Biology, 20.09.2019 20:30

Mathematics, 20.09.2019 20:30



Social Studies, 20.09.2019 20:30




History, 20.09.2019 20:30


Mathematics, 20.09.2019 20:30




History, 20.09.2019 20:30

Mathematics, 20.09.2019 20:30

History, 20.09.2019 20:30

Biology, 20.09.2019 20:30