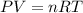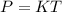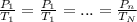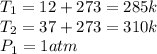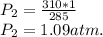Answers: 3
Another question on Physics

Physics, 21.06.2019 16:30
You throw a baseball directly upward at time t = 0 at an initial speed of 12.3 m/s. what is the maximum height the ball reaches above where it leaves your hand? at what times does the ball pass through half the maximum height? ignore air resistance and take g = 9.80 m/s2.
Answers: 1

Physics, 21.06.2019 22:30
How have the competing explanations' experiments on atoms affected the development of the atomic model?
Answers: 1

Physics, 21.06.2019 22:50
Aplane takes off from an airport and flies to town a, located d1 = 215 km from the airport in the direction 20.0° north of east. the plane then flies to town b, located d2 = 230 km at 30.0° west of north from town a. use graphical methods to determine the distance and direction from town b to the airport. (enter the distance in km and the direction in degrees south of west.)
Answers: 2

Physics, 22.06.2019 00:40
Aballet student who learns with the of his instructor is demonstrating learning.
Answers: 3
You know the right answer?
If the air starts at a pressure of 1.0 atm, and you hold the volume of your lungs constant (a good a...
Questions

Mathematics, 03.11.2019 22:31

Biology, 03.11.2019 22:31

Mathematics, 03.11.2019 22:31

History, 03.11.2019 22:31

Biology, 03.11.2019 22:31

Mathematics, 03.11.2019 22:31

Mathematics, 03.11.2019 22:31



Mathematics, 03.11.2019 22:31


Chemistry, 03.11.2019 22:31