
Physics, 12.12.2019 20:31 Serenitybella
A1.00 kg chunk of an unknown metal that has been in boiling water for several minutes is quickly dropped into an insulating styrofoam beaker containing 1.00 kg of water at 18.0°c. after gently stirring for 5.00 minutes, you observe that the water's temperature has reached a constant value of 22.0°c . the specific heat capacity of water is cw = 4190 j/(kg * k) .
1. assuming that the styrofoam absorbs a negligibly small amount of heat and that no heat was lost to the surroundings, what is the specific heat capacity of the metal cm?
2. find the temperature change of the water, δtw, and that of the metal, δtm. express your answers in kelvins separated by a comma.

Answers: 2
Another question on Physics

Physics, 22.06.2019 02:00
How does the amount of energy required to hold each proton and neutron in the nucleus compare to the energy released when they are removed?
Answers: 3

Physics, 22.06.2019 14:20
The energy released by a nuclear fusion reaction is produced when
Answers: 1

Physics, 22.06.2019 15:30
Two point charges are separated by a certain distance. how does the strength of the electric field produced by the first charge, at the position of the second charge, change if the second charge is doubled? a. the field does not change b. the field strength decreases by half. c. the field strength doubles d. the field strength quadruples
Answers: 1

Physics, 22.06.2019 20:30
Ahockey player of mass 82 kg is traveling north with a velocity of 4.1 meters per second he collides with the 76 kg player traveling east at 3.4 meters per second if the two players locked together momentarily in what direction will they be going immediately after the collision how fast will they be moving
Answers: 2
You know the right answer?
A1.00 kg chunk of an unknown metal that has been in boiling water for several minutes is quickly dro...
Questions

Mathematics, 18.03.2020 22:08

Mathematics, 18.03.2020 22:08






Mathematics, 18.03.2020 22:08

Mathematics, 18.03.2020 22:08



Biology, 18.03.2020 22:08



Mathematics, 18.03.2020 22:08




Mathematics, 18.03.2020 22:08

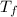 ) = m₂ ce₂ (
) = m₂ ce₂ (

