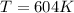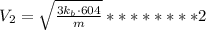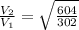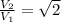Physics, 12.12.2019 21:31 alyssaarnold230
Asample of an ideal gas is in a tank of constant volume. the sample absorbs heat energy so that its temperature changes from 302 k to 604 k. if v1 is the average speed of the gas molecules before the absorption of heat and v2 their average speed after the absorption of heat, what is the ratio v2/v1?
1.v2/v1 = 2.
2.v2/v1 = 4
3.v2/v1 = √2
4.v2/v1 = 2
5.v2/v1 =1/2

Answers: 2
Another question on Physics

Physics, 22.06.2019 00:30
Which is not one of the major climate zones? question 3 options: rain forest polar tropical temperate
Answers: 1

Physics, 22.06.2019 03:30
The solar panels used by mark function because of the photoelectric effect. light shines on the cells causing electrons to be ejected from the metal, which produces an electric current. at night on mars, no light will fall on the solar cells and no electric current will be generated. according to your notes, what type of light is typically needed to cause the photoelectric effect? a)visible b)ultraviolet c)infrared
Answers: 1

Physics, 22.06.2019 05:30
Choose the most likely outcome of this scenario: jen decided to go bike riding without a helmet. while no one is around during her ride, she is thrown from her bike when her wheel goes into a pothole. she is not injured, but she is terrified to get back on her bike. what happens next? a. her physical health is affected even though she wasn't hurt. b. her mental and emotional health are affected because she is afraid to get back on her bike. c. her social health is affected because she is worried her friends saw the fall. d. her overall health is not affected at all by her fall.
Answers: 1

Physics, 22.06.2019 06:40
Use the right-hand rule for magnetic force to determine the charge on the moving particle. this is a charge.
Answers: 1
You know the right answer?
Asample of an ideal gas is in a tank of constant volume. the sample absorbs heat energy so that its...
Questions


Mathematics, 07.04.2020 23:10


History, 07.04.2020 23:10



Mathematics, 07.04.2020 23:10

English, 07.04.2020 23:10


History, 07.04.2020 23:10


Mathematics, 07.04.2020 23:10

Mathematics, 07.04.2020 23:10

English, 07.04.2020 23:10



Mathematics, 07.04.2020 23:10


Computers and Technology, 07.04.2020 23:10

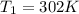
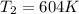
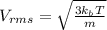
 temperature
temperature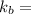 constant
constant