
Physics, 13.12.2019 04:31 kmoo176394
Amonatomic ideal gas that is initially at a pressure of 1.50 x 10^5 pa and has a volume of 0.08 m^3 is compressed adiabatically to a volume of 0.0400 m^3.
(a) what is the final pressure?
(b) how much work is done by the gas?
(c) what is the ratio of the final temperature of the gas to its initial temperature? is the gas heated or cooled by this compression?

Answers: 3
Another question on Physics

Physics, 21.06.2019 20:30
Which of the following is a source of heat for magma formation? magma plumes in the continental crust friction due to divergence friction due to subduction magma plumes in the oceanic crust
Answers: 1

Physics, 22.06.2019 00:40
Aballet student who learns with the of his instructor is demonstrating learning.
Answers: 3

Physics, 22.06.2019 06:30
What are similarities and differences between refraction, reflection, diffraction and absorption?
Answers: 3

Physics, 22.06.2019 11:00
Arowboat passenger uses an oar to push the boat off the dock by exerting a force of 40n for 3.0s. what impulse acts on the boat.
Answers: 1
You know the right answer?
Amonatomic ideal gas that is initially at a pressure of 1.50 x 10^5 pa and has a volume of 0.08 m^3...
Questions

Social Studies, 05.01.2021 16:20


Mathematics, 05.01.2021 16:20








Social Studies, 05.01.2021 16:20


Computers and Technology, 05.01.2021 16:20

Business, 05.01.2021 16:20

Mathematics, 05.01.2021 16:20

History, 05.01.2021 16:20




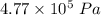 .
.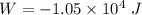 .
.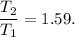 and gas is heated.
and gas is heated.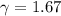 .
.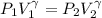 .
.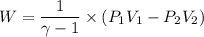 .
.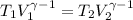 .
.

