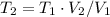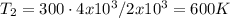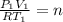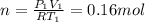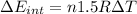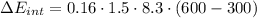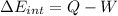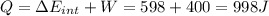Physics, 13.12.2019 17:31 angelread53621
An ideal monatomic gas at a pressure of 2.0×105n/m2 and a temperature of 300 k undergoes a quasi-static isobaric expansion from 2.0×103to4.0×103cm3.
(a) what is the work done by the gas?
(b) what is the temperature of the gas after the expansion?
(c) how many moles of gas are there?
(d) what is the change in internal energy of the gas?
(e) how much heat is added to the gas?

Answers: 1
Another question on Physics


Physics, 22.06.2019 02:50
Ammonia enters the expansion valve of a refrigeration system at a pressure of 10 bar and a temperature of 18oc and exits at 6.0 bar. the refrigerant undergoes a throttling process. determine the temperature, in oc, and the quality of the refrigerant at the exit of the expansion valve.
Answers: 3

Physics, 22.06.2019 06:30
If an atom contains 11 protons in its nucleus, predict the element with the atomic number and electronic configuration. will the number of electrons remain the same during the chemical reaction in such an atom? explain.
Answers: 3

Physics, 22.06.2019 12:00
The mass of a bowling ball is 10 kg and the mass of a ping pong ball is 0.015 kg. if the two objects are 5 meters apart, what is the gravitational force ?
Answers: 1
You know the right answer?
An ideal monatomic gas at a pressure of 2.0×105n/m2 and a temperature of 300 k undergoes a quasi-sta...
Questions


Advanced Placement (AP), 27.07.2019 06:00

Mathematics, 27.07.2019 06:00




Mathematics, 27.07.2019 06:00




Mathematics, 27.07.2019 06:00



Health, 27.07.2019 06:00



Mathematics, 27.07.2019 06:00

Mathematics, 27.07.2019 06:00


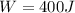
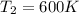
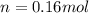
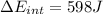
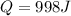
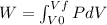
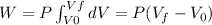
![W=2.0\cdot 10^{5}(4.0\cdot 10^{-3}-2.0\cdot 10^{-3})= 400 [Nm^{2}]](/tpl/images/0417/2115/de6cf.png)
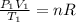 (1)
(1)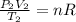 (2)
(2)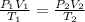 (3)
(3)