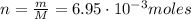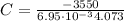At constant volume, the heat of combustion of a particular compound is − 3550.0 kj / mol. when 1.075 g of this compound ( molar mass = 154.74 g / mol ) was burned in a bomb calorimeter, the temperature of the calorimeter, including its contents, rose by 4.073 ∘ c. what is the heat capacity (calorimeter constant) of the calorimeter?

Answers: 1
Another question on Physics

Physics, 22.06.2019 14:00
Often called simply "velocity," this is the velocity of an object at a particular moment in time.
Answers: 1

Physics, 22.06.2019 15:30
Two pans of a balance are 24.1 cm apart. the fulcrum of the balance has been shifted 1.33 cm away from the center by a dishonest shopkeeper. by what percentage is the true weight of the goods being marked up by the shopkeeper? assume the balance has negligible mass. answer in units of %.
Answers: 1

Physics, 22.06.2019 21:10
Select the best definition for wavelength. the height of an oscillating electromagnetic wave the rate at which electromagnetic waves oscillate. the distance between two crests of an electromagnetic wave. the oscillations of electric and magnetic fields select the best definition for frequency. the oscillations of electric and magnetic fields. the height of an oscillating electromagnetic wave. the distance between two crests of an electromagnetic wave. the rate at which electromagnetic waves oscillate
Answers: 1

You know the right answer?
At constant volume, the heat of combustion of a particular compound is − 3550.0 kj / mol. when 1.075...
Questions

Computers and Technology, 13.07.2019 13:30

Mathematics, 13.07.2019 13:30

Mathematics, 13.07.2019 13:30


Mathematics, 13.07.2019 13:30


Mathematics, 13.07.2019 13:30


English, 13.07.2019 13:30


Mathematics, 13.07.2019 13:30

History, 13.07.2019 13:30


History, 13.07.2019 13:30


Biology, 13.07.2019 13:30

Mathematics, 13.07.2019 13:30

History, 13.07.2019 13:30


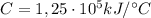
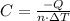 (1)
(1)