
Ablock of metal initially at 25°c is submerged in water initially at 75°c in a coffee cup. which of the following is true?
a. the final temperature of the coffee cup will be less than 20°c.
b. the final temperature of the coffee cup will be higher than 25°c but less than 75°c.
c. the final temperature of the coffee cup will be more than 80°c.
d. the final temperature of the coffee cup will be 80°c.

Answers: 3
Another question on Physics

Physics, 21.06.2019 17:30
The particles of an ice cube at 0°c (represented by the blue circles) come in contact with the particles of juan’s hand at 37°c (represented by the orange circles). which diagram correctly represents the temperature of the two substances and the direction of heat flow?
Answers: 3

Physics, 22.06.2019 03:10
Aphysical change is a change in the size, shape,, or stafe of matter true or false
Answers: 1

Physics, 22.06.2019 15:00
Amoving company collects data about couches they move and displays the data in a table. the force along the ramp, the angle of the ramp, and the height the couch is raised to are shown. which statements correctly compare the work done to move the couches? check all that apply. the same amount of work is done for couches 1 and 3. the same amount of work is done for couches 2 and 3. the work for couch 1 is greater than the work for couch 2. the work for couch 2 is less than the work for couch 3. the work for couch 3 is greater than the work for couch 1. the work for couch 2 is greater than the work for couch 1.
Answers: 1

Physics, 22.06.2019 17:40
A15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°c to 175°c. what is the specific heat capacity of iron?
Answers: 1
You know the right answer?
Ablock of metal initially at 25°c is submerged in water initially at 75°c in a coffee cup. which of...
Questions




Mathematics, 24.06.2021 03:10


History, 24.06.2021 03:10

Biology, 24.06.2021 03:10

History, 24.06.2021 03:10


SAT, 24.06.2021 03:10



English, 24.06.2021 03:20

English, 24.06.2021 03:20


Mathematics, 24.06.2021 03:20


Social Studies, 24.06.2021 03:20

Mathematics, 24.06.2021 03:20

Mathematics, 24.06.2021 03:20

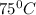 and
and 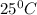 to have thermal equilibrium.
to have thermal equilibrium. 

