
Physics, 13.12.2019 21:31 kakalli9999
Agas in a cylinder is held at a constant pressure of 1.80 * 105 pa and is cooled and compressed from 1.70 m3 to 1.20 m3. the internal energy of the gas decreases by 1.40 * 105 j.
(a) find the work done by the gas.
(b) find the absolute value |q| of the heat flow into or out of the gas, and state the direction of the heat flow.
(c) does it matter whether the gas is ideal? why or why not?

Answers: 2
Another question on Physics

Physics, 21.06.2019 19:30
A500g object falls off a cliff and losers 100 j from its gravitational potential energy store. if the gravitational field strength g=9.8n/kg, how high is the cliff?
Answers: 1

Physics, 22.06.2019 00:30
In positive numbers less than 1, the zeros between the decimal point and non zero are significant
Answers: 2

Physics, 22.06.2019 02:00
Which safety measures should you follow during a thunderstorm? check all that apply. (a) avoid touching anything that conducts electricity. (b) avoid touching a person who has been struck by lightning. (c) go outside. (d) keep your computer turned off. (e) stay out of water. (f) stay inside.
Answers: 2

Physics, 22.06.2019 04:10
Calculate the work done by an external agent during an isothermal compression of 1.00 mol of oxygen from a volume of 22.4 l at 10∘c and 1.0 atm pressure to 16.8l
Answers: 2
You know the right answer?
Agas in a cylinder is held at a constant pressure of 1.80 * 105 pa and is cooled and compressed from...
Questions



Social Studies, 05.07.2019 17:30

Health, 05.07.2019 17:30

Mathematics, 05.07.2019 17:30


History, 05.07.2019 17:30

History, 05.07.2019 17:30

Geography, 05.07.2019 17:30


History, 05.07.2019 17:30


Mathematics, 05.07.2019 17:30


History, 05.07.2019 17:30

Biology, 05.07.2019 17:30



Mathematics, 05.07.2019 17:30

Mathematics, 05.07.2019 17:30

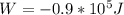 and it is negative because the exterior is doing work on the gas.
and it is negative because the exterior is doing work on the gas.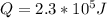 and is directed out of the gas, as this is being cooled and compressed.
and is directed out of the gas, as this is being cooled and compressed.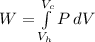
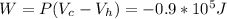
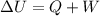
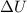 is the internal energy difference,
is the internal energy difference, 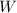 is the calculated work, and
is the calculated work, and 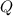 is the heat flow that we wish to know, then
is the heat flow that we wish to know, then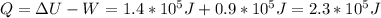
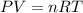 , and
, and  is constant.
is constant.

