
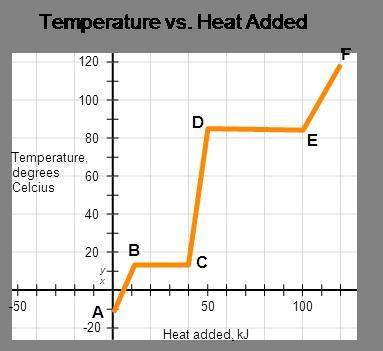
The represented substance has the following heat capacities, enthalpy of fusion, and enthalpy of vaporization.
cp, solid = 3.2 j/(g•°c); cp, liquid = 5.3 j/(g•°c);
cp, vapor = 8.9 j/(g•°c); δhfus = 4.5 kj/mol;
δhvap = 8.6 kj/mol
calculate the total energy input required to accomplish vaporization of one mole of substance at 15°c. the molar mass of the substance is 120.0 g/mol.

Answers: 2
Another question on Physics

Physics, 21.06.2019 16:00
Six solutions are made, each with a y concentration of 1.0 μm and varying concentrations of ab as shown below. based on the concentrations, rank the solutions in decreasing order of reaction rate.
Answers: 2

Physics, 22.06.2019 12:00
An architect plans to use solar energy to heat the next house he designs. what principle of absorption and infrared energy can be applied to the design of the new house? how could she apply to those principals?
Answers: 2

Physics, 22.06.2019 17:30
Which basic property determines a colored light's placement on the spectrum of visible light? a. speed b. frequency c. amplitude d. velocity
Answers: 3

Physics, 22.06.2019 18:30
Arailroad car collides with and sticks to an identical railroad car that is initially at rest. after the collision, the total kinetic energy of the two cars is a) the same as before. b) half as much as before. c) one third as much as before. d) one fourth as much as before. e) twice as much as before.
Answers: 1
You know the right answer?
The represented substance has the following heat capacities, enthalpy of fusion, and enthalpy of vap...
Questions

Mathematics, 21.05.2021 01:40





Mathematics, 21.05.2021 01:40


Mathematics, 21.05.2021 01:40

Mathematics, 21.05.2021 01:40


Mathematics, 21.05.2021 01:40

Mathematics, 21.05.2021 01:40

Mathematics, 21.05.2021 01:40

Biology, 21.05.2021 01:40

Mathematics, 21.05.2021 01:40

Arts, 21.05.2021 01:40

History, 21.05.2021 01:40

Physics, 21.05.2021 01:40


Mathematics, 21.05.2021 01:40



