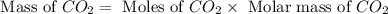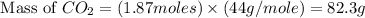Problem page gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 28. g of ethane is mixed with 190. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to significant digits. clears your work. undoes your last action. provides information about entering answers. g

Answers: 1
Another question on Physics

Physics, 22.06.2019 06:30
At very high pressures, gases become and will eventually a) more dense; become hotter b) more dense; change to a liquid or solid c) less dense; combust d) less dense; turn into a liquid
Answers: 1

Physics, 22.06.2019 11:00
Although longitudinal waves can travel through all media types what is the disadvantage of this type of wave transmission? a) he efficiency of he wave transmission is greatly diminished as the media becomes less dense b) the oscillation of one set of particles has a huge impact on neighboring particles c) particles become too close together as they bump into each other making it more likely to hit other particles down the line
Answers: 2


Physics, 22.06.2019 19:30
How many electrons in an atom could have these sets of quantum numbers? =3n=3 electronselectrons =4,ℓ=2n=4,ℓ=2 electronselectrons =6,ℓ=ℓ=−1n=6,ℓ=2,mℓ=−1
Answers: 3
You know the right answer?
Problem page gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gas...
Questions

Mathematics, 16.10.2020 07:01


Social Studies, 16.10.2020 07:01


History, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01


Biology, 16.10.2020 07:01


History, 16.10.2020 07:01




Mathematics, 16.10.2020 07:01

History, 16.10.2020 07:01


History, 16.10.2020 07:01


Biology, 16.10.2020 07:01

Law, 16.10.2020 07:01

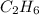 = 28 g
= 28 g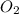 = 190 g
= 190 g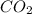 = 44 g/mole
= 44 g/mole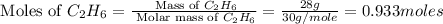
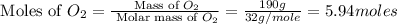
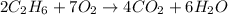
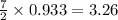 moles of
moles of 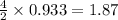 moles of
moles of