
At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. sufficient pcl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250 °c. calculate the pressure of pcl5 after the system has reached equilibrium.
a. 1.50 atm
b. 1.24 atm
c. 4.24 atm
d. 0.94 atm
e. 1.12 atm

Answers: 3
Another question on Physics

Physics, 21.06.2019 21:00
While listening to operatic solos, musicians process the lyrics and the tunes in separate brain areas. this most clearly illustrates the functioning of different
Answers: 1

Physics, 21.06.2019 22:30
Acricket ball of 70g moving with a velocity of 0.5 m/s is stopped by a player in 0.5s what is the force applied to stop the ball
Answers: 1

Physics, 22.06.2019 04:30
Which of the following is not a characteristic of s waves? a. travel slower than p waves. b. cannot be detected in locations more than 105° from an earthquake’s epicenter. c. travel through solids and liquids. d. only affect coastal regions.
Answers: 2

Physics, 22.06.2019 10:40
When the magnetic domains in a material can be aligned, but eventually drift out of alignment, the material is
Answers: 2
You know the right answer?
At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. suffici...
Questions

Mathematics, 12.04.2021 08:40

Mathematics, 12.04.2021 08:40


Mathematics, 12.04.2021 08:40

Advanced Placement (AP), 12.04.2021 08:40



Spanish, 12.04.2021 08:40


Mathematics, 12.04.2021 08:40


Mathematics, 12.04.2021 08:40


Mathematics, 12.04.2021 08:40

Mathematics, 12.04.2021 08:40



Mathematics, 12.04.2021 08:40


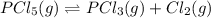
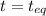 2.74-x x x
2.74-x x x
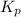 for the given reaction follows:
for the given reaction follows:
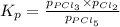
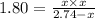
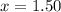
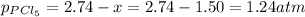
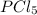 at equilibrium is 1.24 atm
at equilibrium is 1.24 atm


