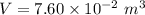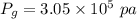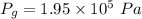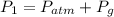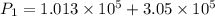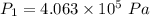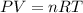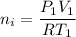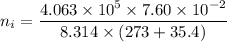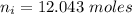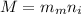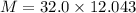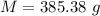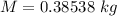Physics, 17.12.2019 04:31 AnActualTrashcan
Awelder using a tank of volume 7.60×10−2 m3 fills it with oxygen (with a molar mass of 32.0 g/mol ) at a gauge pressure of 3.05×105 pa and temperature of 35.4 ∘c. the tank has a small leak, and in time some of the oxygen leaks out. on a day when the temperature is 20.8 ∘c, the gauge pressure of the oxygen in the tank is 1.95×105 pa. find the initial mass of oxygen. (in kg)

Answers: 3
Another question on Physics

Physics, 21.06.2019 16:50
Identify the arrows that show the correct direction of heat transfer. 66°f 112°f 98°f
Answers: 2

Physics, 22.06.2019 04:40
Argon is adiabatically compressed from an initial volume of 16 liters to a final volume of 2 liters. by what factor do the following quantities change? do they increase or decrease? (a) the rms speed (b) the thermal energy of the gas (c) the molar specific heat cv (d) the pressure
Answers: 3

Physics, 22.06.2019 16:20
Specific heat refers to the amount of heat required to change 1 gram of a substance by degree(s) celsius
Answers: 1

Physics, 22.06.2019 17:30
Chameleons catch insects with their tongues, which they can rapidly extend to great lengths. in a typical strike, the chameleon's tongue accelerates at a remarkable 220 m/s2 for 20 ms, then travels at constant speed for another 30 ms.
Answers: 1
You know the right answer?
Awelder using a tank of volume 7.60×10−2 m3 fills it with oxygen (with a molar mass of 32.0 g/mol )...
Questions

Mathematics, 05.10.2020 20:01



Engineering, 05.10.2020 20:01

Mathematics, 05.10.2020 20:01





Mathematics, 05.10.2020 20:01


Mathematics, 05.10.2020 20:01





History, 05.10.2020 20:01



Mathematics, 05.10.2020 21:01