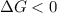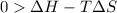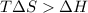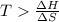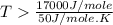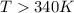One might be tempted to say that exothermic processes are always spontaneous since the system is emitting energy (heat) in order to reach a (preferred) lower energy state. however, as we have just investigated, the spontaneous process for polymers is endothermic. this reveals that we must consider entropy changes when determining the nature of spontaneity. the most probable configuration of a system and its surroundings, naturally, is the one that will be observed. the condition for spontaneity can be recast using the concept of the free energy of the system, where a change in free energy results both from changes in the enthalpy (which includes internal potential and kinetic energies) and the entropy (the number of states accessible to the system). δ g = δ h − t δ s.
an unknown chemical reaction undergoes an enthalpy change of δ h =17 kj/mol while the entropy increases by δ s =50 j/(mol * k).
above what temperature (in kelvin) does this reaction occur spontaneously?

Answers: 1
Another question on Physics

Physics, 22.06.2019 15:40
Apotter's wheel moves uniformly from rest to an angular speed of 0.20 rev/s in 32.0 s. (a) find its angular acceleration in radians per second per second. rad/s2 (b) would doubling the angular acceleration during the given period have doubled final angular speed?
Answers: 1

Physics, 23.06.2019 00:00
Boiling water is an example of physical change. true or false
Answers: 2

Physics, 23.06.2019 00:30
Which of the following statements accurately describes the sign of the work done on the box by the force of the push? a. positive b. negative c. zero
Answers: 3

Physics, 23.06.2019 00:30
Which of the following statements are true for an isothermal process? check all that apply. - during an isothermal process, the work done by the gas equals the heat added to the gas. - during an isothermal process, the internal energy of the system changes. - an isothermal process is carried out at constant temperature. - an isothermal process is carried out at constant pressure. - an isothermal process is carried out at constant volume.
Answers: 1
You know the right answer?
One might be tempted to say that exothermic processes are always spontaneous since the system is emi...
Questions


Biology, 28.11.2020 18:10



History, 28.11.2020 18:10


Mathematics, 28.11.2020 18:10

Mathematics, 28.11.2020 18:10

Mathematics, 28.11.2020 18:10

Medicine, 28.11.2020 18:10

Mathematics, 28.11.2020 18:10

Mathematics, 28.11.2020 18:10

Chemistry, 28.11.2020 18:20

Advanced Placement (AP), 28.11.2020 18:20


Mathematics, 28.11.2020 18:20

Arts, 28.11.2020 18:20

Mathematics, 28.11.2020 18:20

English, 28.11.2020 18:20

Geography, 28.11.2020 18:20

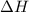 = 17 KJ/mole = 17000 J/mole
= 17 KJ/mole = 17000 J/mole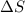 = 50 J/mole.K
= 50 J/mole.K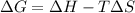
 is negative or we can say that the value of
is negative or we can say that the value of