
Physics, 19.12.2019 06:31 kaywendel2008
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula: =e−ryn2 in this equation ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron. (you can find the value of the rydberg energy using the data button on the aleks toolbar.) calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with =n10 to an orbital with =n8. round your answer to 3 significant digits.

Answers: 3
Another question on Physics

Physics, 21.06.2019 14:00
How much heat is required to convert 0.3 kg of ice at 0°c to water at the same temperature
Answers: 1

Physics, 22.06.2019 08:00
1.what is where an object is located 2. what is how fast an objects speed is changing 3. what is how fast an object is moving
Answers: 1

Physics, 22.06.2019 13:20
Which of the following is the main energy source for the sun? a. fission of hydrogen b. fusion of hydrogen c. fusion of helium d. fission of iron
Answers: 2

Physics, 22.06.2019 15:10
Auniform crate c with mass mc is being transported to the left by a forklift with a constant speed v1. what is the magnitude of the angular momentum of the crate about point a, that is, the point of contact between the front tire of the forklift and the ground
Answers: 3
You know the right answer?
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula: =e−ryn2 in...
Questions

Mathematics, 09.06.2021 04:00


History, 09.06.2021 04:00


Mathematics, 09.06.2021 04:00



Mathematics, 09.06.2021 04:00


Social Studies, 09.06.2021 04:00







Social Studies, 09.06.2021 04:00


Arts, 09.06.2021 04:00

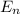 = - k²e² / 2m (1 / n²)
= - k²e² / 2m (1 / n²)
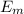 = - k²e² / 2m (1 /
= - k²e² / 2m (1 / 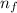 ² -1 /
² -1 / 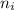 ²)
²)


