
Physics, 19.12.2019 19:31 tristanlindor5908
Determione of the lines in the balmer series of the hydrogen atom emission spectrum is at 397 nm. it results from a transition from an upper energy level to n=2. what is the principal quantum number of the upper level? ne the initial energy level, ninitial from which the electron in the hydrogen relaxed from to the final energy level, nfinal , 4, if the emitted light has a frequency equal to 74 x 1012 (1/sec). is this wavelength in the visible region of the electromagnetic spectrum?

Answers: 3
Another question on Physics


Physics, 22.06.2019 11:00
Alarge box of mass m is pulled across a horizontal frictionless surface by a horizontal rope with tension t. a small box of mass m sits on top of the large box. the coefficients of static and
Answers: 1


Physics, 22.06.2019 14:40
On a geologic map, if the contacts between sedimentary rock units form a bull’s-eye pattern of concentric circles, with the youngest unit in the center, the underlying structure is a(n)
Answers: 3
You know the right answer?
Determione of the lines in the balmer series of the hydrogen atom emission spectrum is at 397 nm. it...
Questions

Engineering, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01


English, 20.10.2020 02:01

English, 20.10.2020 02:01








Mathematics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01





Mathematics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01

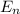 = - 13.606 1/n²
= - 13.606 1/n²
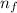 ² - 1/n₀²)
² - 1/n₀²)
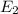 -
- 

