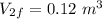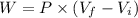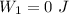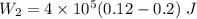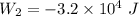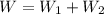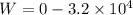Physics, 20.12.2019 18:31 ajbrock1004
An ideal gas goes through the following two-step process. 1) the container holding the gas has a fixed volume of 0.200 m3 while the pressure of the gas increases from 3.00×105 pa to 4.00×105 pa . 2) the container holding the gas is then compressed to a volume of 0.120 m3 while maintaining a constant pressure of 4.00×105 pa .a) what is the total work done by the gas for this two-step process?

Answers: 2
Another question on Physics


Physics, 22.06.2019 10:00
Your town is considering building a biodiesel power plant describe at least two advantages and two disadvantages
Answers: 1


Physics, 22.06.2019 12:50
Match each vocabulary term to its definition. 1. electrons neutral subatomic particles found in the nucleus of the atom 2. neutron lowest energy position of an electron in an atom 3. photon the path of an electron around the nucleus of an atom 4. ground state negatively charged, subatomic particles 5. protons packet of energy of specific size 6. element substance with only one type of atom 7. orbital positively charged, subatomic particles found in the nucleus of the atom
Answers: 1
You know the right answer?
An ideal gas goes through the following two-step process. 1) the container holding the gas has a fix...
Questions



History, 26.07.2019 23:30

History, 26.07.2019 23:30


Mathematics, 26.07.2019 23:30










Computers and Technology, 26.07.2019 23:30

Mathematics, 26.07.2019 23:30



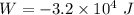
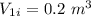 Initial pressure,
Initial pressure, 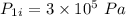 Final pressure,
Final pressure, 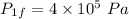
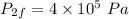 Final volume,
Final volume,