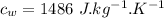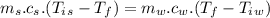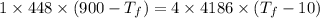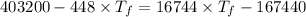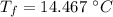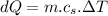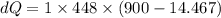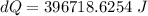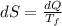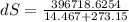Physics, 24.12.2019 06:31 haleyrene3924
A1.00-kg iron horseshoe is taken from a forge at 900∘c and dropped into 4.00 kg of water at 10.0∘c. assuming that no energy is lost by heat to the surroundings, determine the total entropy change of the horseshoe-plus-water system.

Answers: 1
Another question on Physics


Physics, 22.06.2019 06:30
At very high pressures, gases become and will eventually a) more dense; become hotter b) more dense; change to a liquid or solid c) less dense; combust d) less dense; turn into a liquid
Answers: 1

Physics, 22.06.2019 10:00
Asap and show ! a 14 kg rock starting from rest free falls through a distance of 5.0 m with no air resistance. find the momentum change of the rock caused by its fall and the resulting change in the magnitude of earths velocity. earth mass is 6.0 * 10^24 kg. show your work assuming the rock earth system is closed.
Answers: 2

Physics, 22.06.2019 18:10
Arefrigerator uses r-134a as the working fluid and operates on the ideal vapor-compression refrigeration cycle except for the compression process. the refrigerant enters the evaporator at 120 kpa with a quality of 34 percent and leaves the compressor at 70°c. if the compressor consumes 450 w of power, determine (a) the mass flow rate of the refrigerant, (b) the condenser pressure, and (c) the cop of the refrigerator. (0.00644 kg/s, 800 kpa, 2.03)
Answers: 1
You know the right answer?
A1.00-kg iron horseshoe is taken from a forge at 900∘c and dropped into 4.00 kg of water at 10.0∘c....
Questions




















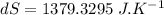
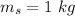 initial temperature of iron,
initial temperature of iron, 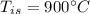 mass of water,
mass of water, 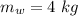 initial temperature of water,
initial temperature of water, 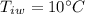
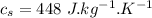 Specific heat of water,
Specific heat of water,