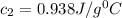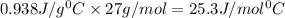Physics, 24.12.2019 21:31 jamiezanfardino1464
A23.5 g piece of aluminum metal is initially at 100.0°c. it is dropped into a coffee cup-calorimeter containing 130.0 g of water at a temperature of 23.0°c. after stirring, the final temperature of both copper and water is 26.0°c. assuming no heat losses, and that the specific heat capacity of water is 4.184 j/(g·°c), what is the molar heat capacity of aluminum, cm(al)?

Answers: 3
Another question on Physics

Physics, 22.06.2019 01:00
15. give an example for some particles or waves that are moving faster than light in everyday life 16. what is a laser? 17. what is an oscilloscope? 18. what does it means practically that nothing is faster than light in vacuum? 19. what is vacuum?
Answers: 2


Physics, 22.06.2019 11:20
The ultracentrifuge is an important tool for separating and analyzing proteins. because of the enormous centripetal accelerations, the centrifuge must be carefully balanced, with each sample matched by a sample of identical mass on the opposite side. any difference in the masses of opposing samples creates a net force on the shaft of the rotor, potentially leading to a catastrophic failure of the apparatus. suppose a scientist makes a slight error in sample preparation and one sample has a mass 10 mg larger than the opposing sample. if the samples are 12 cm from the axis of the rotor and the ultracentrifuge spins at 70,000 rpm, what is the magnitude of the net force on the rotor due to the unbalanced samples? ( be thorough on your answer)
Answers: 3

You know the right answer?
A23.5 g piece of aluminum metal is initially at 100.0°c. it is dropped into a coffee cup-calorimeter...
Questions




Mathematics, 12.09.2021 17:40


World Languages, 12.09.2021 17:40

Mathematics, 12.09.2021 17:40







Mathematics, 12.09.2021 17:40



English, 12.09.2021 17:40

Mathematics, 12.09.2021 17:40

Mathematics, 12.09.2021 17:40

English, 12.09.2021 17:40

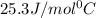
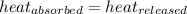
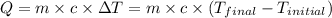
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0432/0719/09236.png) .................(1)
.................(1)
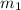 = mass of water = 130.0 g
= mass of water = 130.0 g
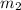 = mass of aluminiunm = 23.5 g
= mass of aluminiunm = 23.5 g
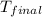 = final temperature
=
= final temperature
= 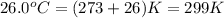
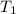 = temperature of water =
= temperature of water = 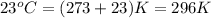
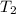 = temperature of aluminium =
= temperature of aluminium = 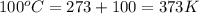
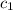 = specific heat of water=
= specific heat of water= 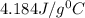
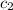 = specific heat of aluminium= ?
= specific heat of aluminium= ?![130.0\times 4.184\times (299-296)=-[23.5\times c_2\times (299-373)]](/tpl/images/0432/0719/5591a.png)