
How much heat energy is required to convert 93.4 g of solid ethanol at − 114.5 ° c to gasesous ethanol at 149.8 ° c ? the molar heat of fusion of ethanol is 4.60 kj/mol , and its molar heat of vaporization is 38.56 kj/mol . ethanol has a normal melting point of − 114.5 ° c and a normal boiling point of 78.4 ° c . the specific heat capacity of liquid ethanol is 2.45 j / g ⋅ ° c , and that of gaseous ethanol is 1.43 j / g ⋅ ° c .

Answers: 3
Another question on Physics

Physics, 22.06.2019 12:10
Light traveling in water, nwater = 1.33, strikes a plastic block at an angle of incidence of 51.4°; part of the beam is reflected and part is refracted. if the index of refraction of the plastic is 2.0, what is the angle made by the reflected and refracted beams?
Answers: 2

Physics, 22.06.2019 15:00
What happens when a rubber rod is rubbed with a piece of fur?
Answers: 1

Physics, 22.06.2019 16:00
In which of the following is positive work done by a person on a suitcase
Answers: 1

Physics, 22.06.2019 16:40
Acapacitor is storing energy of 3 joules with a voltage of 50 volts across its terminals. a second identical capacitor of the same value is storing energy of 1 joule. what is the voltage across the terminals of the second capacitor?
Answers: 3
You know the right answer?
How much heat energy is required to convert 93.4 g of solid ethanol at − 114.5 ° c to gasesous ethan...
Questions


Biology, 14.12.2020 18:40

Computers and Technology, 14.12.2020 18:40

English, 14.12.2020 18:40

Advanced Placement (AP), 14.12.2020 18:40

Mathematics, 14.12.2020 18:40

Advanced Placement (AP), 14.12.2020 18:40

Mathematics, 14.12.2020 18:40

Chemistry, 14.12.2020 18:40

Mathematics, 14.12.2020 18:40

English, 14.12.2020 18:40


Mathematics, 14.12.2020 18:40


Physics, 14.12.2020 18:40

Mathematics, 14.12.2020 18:40


SAT, 14.12.2020 18:40


Mathematics, 14.12.2020 18:40

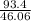 = 2.02 moles
= 2.02 moles

