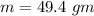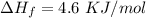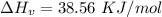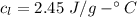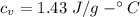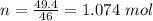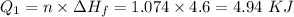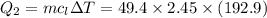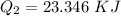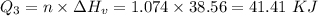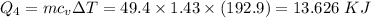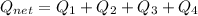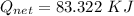Physics, 08.01.2020 02:31 bwright142
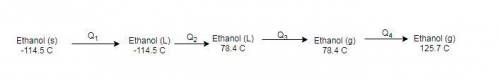
How much heat energy is required to convert 49.4 g of solid ethanol at − 114.5 ° c to gasesous ethanol at 140.7 ° c ? the molar heat of fusion of ethanol is 4.60 kj/mol , and its molar heat of vaporization is 38.56 kj/mol . ethanol has a normal melting point of − 114.5 ° c and a normal boiling point of 78.4 ° c . the specific heat capacity of liquid ethanol is 2.45 j / g ⋅ ° c , and that of gaseous ethanol is 1.43 j / g ⋅ ° c .

Answers: 3
Another question on Physics

Physics, 22.06.2019 04:30
In a system, when potential energy decreases, then entropy also decreases. true false
Answers: 3

Physics, 22.06.2019 15:30
Ineed ! using proper grammar, spelling, and punctuation, write at least one 5 sentence paragraph describing 3 ways we use the elements of the electromagnetic spectrum (ems) in our everyday lives.
Answers: 1

Physics, 22.06.2019 17:30
Convection currents are caused by differences in what two things?
Answers: 1

Physics, 22.06.2019 18:30
Anonzero net force acts on a particle and does work. which one of the following statements is true? the kinetic energy of the particle changes, but the speed of the particle does not change. the kinetic energy of the particle does not change, but the speed of the particle does change. the kinetic energy of the particle changes, but the velocity of the particle does not change. the kinetic energy and the speed of the particle change, but the velocity of the particle does not change. the kinetic energy, speed, and velocity of the particle change.
Answers: 1
You know the right answer?
How much heat energy is required to convert 49.4 g of solid ethanol at − 114.5 ° c to gasesous ethan...
Questions



Computers and Technology, 21.07.2019 07:00




English, 21.07.2019 07:00

Mathematics, 21.07.2019 07:00

History, 21.07.2019 07:00

History, 21.07.2019 07:00

History, 21.07.2019 07:00



Biology, 21.07.2019 07:00


Computers and Technology, 21.07.2019 07:00

History, 21.07.2019 07:00

Mathematics, 21.07.2019 07:00

Physics, 21.07.2019 07:00