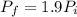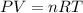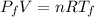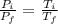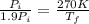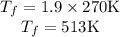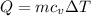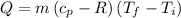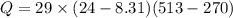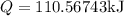Physics, 18.01.2020 00:31 RickyGotFanz4867
Asealed tank contains 29 moles of an ideal gas at an initial temperature of the pressure of the gas is increased until the final pressure equals 1.9 times the initial pressure. the heat capacity at constant pressure of the gas is what is the heat absorbed by the gas? let the ideal-gas constant r = 8.314 j/(mol • k).
7.0 kj
170 kj
230 kj
110 kj
-52 kj

Answers: 2
Another question on Physics

Physics, 22.06.2019 06:40
Use the right-hand rule for magnetic force to determine the charge on the moving particle. this is a charge.
Answers: 1

Physics, 22.06.2019 16:30
Acoil suspended freely, points in some direction when no current is passed through it . can you tell what will happen when a current is passed though it?
Answers: 3

Physics, 23.06.2019 00:30
Which of the following are forms of phase changes a. freezing b. condensation c. heating d. exchanging
Answers: 2

Physics, 23.06.2019 02:10
The molecular mass of a gas is (select all that apply) a. independent of the type of gas b. the same number as the atomic (or molecular) mass, but measured in grams c. dependent on the temperature d. the mass of a mole of the gas
Answers: 1
You know the right answer?
Asealed tank contains 29 moles of an ideal gas at an initial temperature of the pressure of the gas...
Questions

English, 21.10.2020 04:01



Mathematics, 21.10.2020 04:01

Chemistry, 21.10.2020 04:01

Computers and Technology, 21.10.2020 04:01

Mathematics, 21.10.2020 04:01





Mathematics, 21.10.2020 04:01


History, 21.10.2020 04:01



Mathematics, 21.10.2020 04:01

English, 21.10.2020 04:01

Mathematics, 21.10.2020 04:01

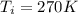
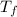 , the initial pressure will be
, the initial pressure will be 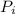 and the final pressure will be,
and the final pressure will be,