Physics, 23.01.2020 07:31 anastasiasam8996
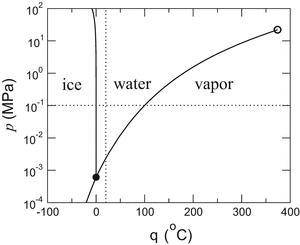
2.09 j> g °c, and that of steam is 2.01 j> g °c. 72. how much heat (in kj) is evolved in converting 1.00 mol of at - 10.0 °c, to steam at 110.0 °c? the heat capacity of ice is ## 2.01 j> g °c, and that of ice is 2.09 j> g °c. phase diagrams steam at 145 °c to ice at - 50 °c? the heat capacity of steam is ##

Answers: 3
Another question on Physics

Physics, 22.06.2019 04:00
Amodel rocket with a mass of 0.212 kg is launched into the air with an initial speed of 84 m/s. how much kinetic energy will the rocket have at a height of 214 m? assume there is no wind resistance. 634 j 303 j
Answers: 2

Physics, 22.06.2019 09:50
Gordon is going for a run through the park, but it is cold outside. the low outside temperature could affect his personal safety.
Answers: 1

Physics, 22.06.2019 11:00
Consider a system to be two train cars traveling toward each other. what is the total momentum of the system before the train cars collide? kg • what must the total momentum of the system be after the train cars collide? kg •
Answers: 2

Physics, 22.06.2019 21:00
The amount of work done is determined by 2 factors. describe an example of work (using the scientific definition) list the 2 factors that determine the amount of work done.
Answers: 2
You know the right answer?
2.09 j> g °c, and that of steam is 2.01 j> g °c. 72. how much heat (in kj) is evolved in conve...
Questions


History, 22.01.2021 07:30

Mathematics, 22.01.2021 07:30

Mathematics, 22.01.2021 07:30



Mathematics, 22.01.2021 07:30




Social Studies, 22.01.2021 07:30




Mathematics, 22.01.2021 07:30

Physics, 22.01.2021 07:30


Mathematics, 22.01.2021 07:30