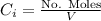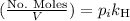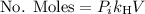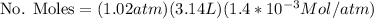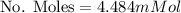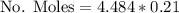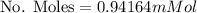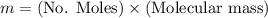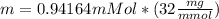Physics, 28.01.2020 20:48 Rodrigo6379
Calculate the mass of oxygen (in mg) dissolved in a 3.14 l bucket of water exposed to a pressure of 1.02 atm of air. assume the mole fraction of oxygen in air to be 0.21 and the henry's law constant for oxygen in water at this temperature to be 1.4 × 10-3 m/atm o2. (enter your value using three significant figures.)

Answers: 3
Another question on Physics

Physics, 22.06.2019 04:00
Acompound machine is also called a machine. a. force b. simple c. complex d. directional
Answers: 1

Physics, 22.06.2019 14:10
The number of passengers who arrive at the platform of a subway station for the 10 am train is a random variable with a mean of 120 and a variance of 16. find the lower bound of the probability that there will be between 100 and 140 passengers (round off to second decimal place).
Answers: 3


Physics, 23.06.2019 12:00
Awave is propagating from left to right in a medium. the particles in the medium are also vibrating from left to right. what kind of wave does this describe? a. longitudinal wave b. transverse wave c. mechanical wave d. electromagnetic wave
Answers: 2
You know the right answer?
Calculate the mass of oxygen (in mg) dissolved in a 3.14 l bucket of water exposed to a pressure of...
Questions








Mathematics, 08.08.2019 23:10


History, 08.08.2019 23:10



Mathematics, 08.08.2019 23:10



Social Studies, 08.08.2019 23:10




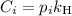
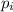 is the partial pressure of the gas.
is the partial pressure of the gas.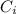 is the concentration of the gas (solubility).
is the concentration of the gas (solubility).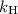 is Henry's constant, which depends on the nature of the gas, the temperature and the liquid.
is Henry's constant, which depends on the nature of the gas, the temperature and the liquid.