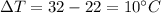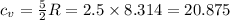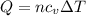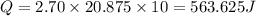Answers: 3
Another question on Physics

Physics, 22.06.2019 11:00
Although longitudinal waves can travel through all media types what is the disadvantage of this type of wave transmission? a) he efficiency of he wave transmission is greatly diminished as the media becomes less dense b) the oscillation of one set of particles has a huge impact on neighboring particles c) particles become too close together as they bump into each other making it more likely to hit other particles down the line
Answers: 2

Physics, 22.06.2019 12:20
Aball with a mass of 275 g is dropped from rest, hits the floor and rebounds upward. if the ball hits the floor with a speed of 2.40 m/s and rebounds with a speed of 1.70 m/s, determine the following. (a) magnitude of the change in the ball's momentum (let up be in the positive direction.)
Answers: 1

Physics, 22.06.2019 13:10
A0.750 kg aluminum pan is removed from the stove and plunged into a sink filled with 10.0 kg of water at 293 k. the water temperature quickly rises to 297 k. what was the initial temperature of the aluminum pan? the specific heat of aluminum is ca = 900 j/(kgk) and water is cw = 4190 j/(kgk)
Answers: 3

Physics, 22.06.2019 15:00
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2,000 j 1: write the equation2: list out your known variables 3: plug the numbers into the equations 4: solve 5: write your solution statement that includes initial energy and final energy added you so much!
Answers: 2
You know the right answer?
How much heat (in j) must be added to raise the temperature of 2.70 mol of air from 22.0°c to 32.0°c...
Questions

Mathematics, 16.01.2020 02:31

Mathematics, 16.01.2020 02:31



Physics, 16.01.2020 02:31


Physics, 16.01.2020 02:31


Physics, 16.01.2020 02:31

Geography, 16.01.2020 02:31


Computers and Technology, 16.01.2020 02:31

Mathematics, 16.01.2020 02:31

Mathematics, 16.01.2020 02:31

History, 16.01.2020 02:31




Mathematics, 16.01.2020 02:31