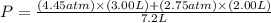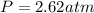Physics, 29.01.2020 05:44 elexiafloyd
You have a 3.00-liter container filled with n₂ at 25°c and 4.45 atm pressure connected to a 2.00-liter container filled with ar at 25°c and 2.75 atm pressure. a stopcock connecting the containers is opened and the gases are allowed to equilibrate between the two containers. what is the final pressure in the two containers if the temperature remains at 25°c? assume ideal behavior.

Answers: 1
Another question on Physics

Physics, 21.06.2019 20:50
What is the type of the reaction below? bacl2 + h2so4 → 2 hci + baso4 double replacement synthesis single replacement decomposition
Answers: 1

Physics, 22.06.2019 02:30
Which is an example of gaining a static charge by conduction? a) rubbing a balloon against your hair. b) shuffling your shoes across a carpet. c) bringing a charged rod near an electroscope. d) touching your car on a cold day and getting a shock.
Answers: 1

Physics, 22.06.2019 09:50
Gordon is going for a run through the park, but it is cold outside. the low outside temperature could affect his personal safety.
Answers: 1

You know the right answer?
You have a 3.00-liter container filled with n₂ at 25°c and 4.45 atm pressure connected to a 2.00-lit...
Questions



Health, 10.02.2020 05:38


Mathematics, 10.02.2020 05:38






Biology, 10.02.2020 05:39


Mathematics, 10.02.2020 05:39




Chemistry, 10.02.2020 05:39

History, 10.02.2020 05:39

Mathematics, 10.02.2020 05:39

Mathematics, 10.02.2020 05:39

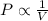
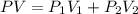
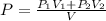
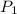 = pressure of N₂ gas = 4.45 atm
= pressure of N₂ gas = 4.45 atm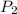 = pressure of Ar gas = 2.75 atm
= pressure of Ar gas = 2.75 atm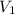 = volume of N₂ gas = 3.00 L
= volume of N₂ gas = 3.00 L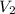 = volume of Ar gas = 2.00 L
= volume of Ar gas = 2.00 L