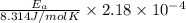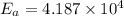Answers: 3
Another question on Physics

Physics, 21.06.2019 17:20
The specific heat of silver is 0.057 calories/gram°c. if 10.0 grams of silver were heated and the temperature of the sample changed by 20.0°c, how many calories of heat energy were absorbed by the sample?
Answers: 1

Physics, 22.06.2019 16:20
What is the mass of the water that is being heated? it requires 2,500 joules to raise a certain amount of water (c = 4.186 jig c) from 20.0°c to 60.0°c. o 159 o 40 g o 63 g o 80 g
Answers: 2

Physics, 22.06.2019 16:30
Each neuron shown in this figure innervates a group of muscle fibers. what is the term for a group of muscle fibers innervated by a single neuron?
Answers: 1

Physics, 22.06.2019 22:00
Which best term to describe a chemical reaction that absorbs energy from its surroundings? a.thermal conductor b.thermal insulator c.endothermic d.exothermic
Answers: 1
You know the right answer?
The rate constant for a reaction at 40.0°C is exactly 3 times that at 20.0°C. Calculate the Arrheniu...
Questions

Mathematics, 21.04.2020 16:15

History, 21.04.2020 16:15

English, 21.04.2020 16:15



Biology, 21.04.2020 16:15

Mathematics, 21.04.2020 16:16

Mathematics, 21.04.2020 16:16



Arts, 21.04.2020 16:16

Health, 21.04.2020 16:16

History, 21.04.2020 16:16

Physics, 21.04.2020 16:16






Mathematics, 21.04.2020 16:16

![ln \frac{k_{2}}{k_{1}} = \frac{E_{a}}{R} [\frac{1}{T_{1}} - \frac{1}{T_{2}}]](/tpl/images/0506/0023/2d1b4.png)
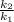 = 3 times.
= 3 times.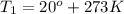
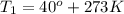
![ln (3) = \frac{E_{a}}{8.314 J/mol K} [\frac{1}{293 K} - \frac{1}{313 K}]](/tpl/images/0506/0023/3da7b.png)