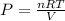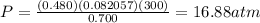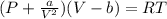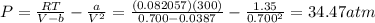Assume that you have 0.480 mol of N2 in a volume of 0.700 L at 300 K .
1. Calculate the...

Physics, 11.02.2020 05:28 eweqwoewoji
Assume that you have 0.480 mol of N2 in a volume of 0.700 L at 300 K .
1. Calculate the pressure in atmospheres using the ideal gas law.
2. Calculate the pressure in atmospheres using the van der Waals equation. For N2 , a=1.35 (L2⋅atm)/mol2 , and b=0.0387 L/mol

Answers: 2
Another question on Physics

Physics, 22.06.2019 07:00
We have a colorless transparent liquid. it looks like water. we seperated it into a solid and a liquid by evaporration and condention was this a chemichal or a physical seperation a. chemical seperation b. physical seperation
Answers: 3

Physics, 22.06.2019 09:30
Which are advantages of renewable resources? check all that apply. renewable energy supplies are completely reliable everywhere. some renewable resources will never be used up. little or no waste is produced by renewable resource plants. electricity can be generated in large quantities. many renewable energy facilities have lower operating costs.
Answers: 1

Physics, 22.06.2019 11:30
If the chemical properties of a substance remain unchanged and appearance or shape of a substance changes it is called a ?
Answers: 1

Physics, 22.06.2019 15:00
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2,000 j 1: write the equation 2: list out your known variables 3: plug the numbers into the equations 4: solve 5: write your solution statement that includes initial energy and final energy added you so much!
Answers: 3
You know the right answer?
Questions






Mathematics, 07.11.2019 01:31






Computers and Technology, 07.11.2019 01:31




Mathematics, 07.11.2019 01:31



English, 07.11.2019 01:31

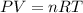 (1)
(1)