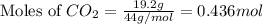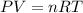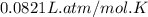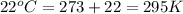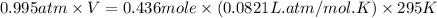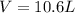Physics, 11.02.2020 23:31 kiarabermudez754
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus. Calculate the expansion work done against a constant external pressure of 0.995 atm and at a constant temperature of 22 degrees C. Assume that the initial volume of dry ice is negligible and that CO2 behaves like an ideal gas.

Answers: 2
Another question on Physics


Physics, 22.06.2019 18:30
4. now look at the green lines you created by connecting the three boiling point data points and the three melting point data points. for each of these lines, describe any trends you see. 5. locate the elements on your periodic table that you circled in green on your graph. what term or description would you use to identify these elements with respect to the periodic table? 7. using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers.
Answers: 2

Physics, 22.06.2019 22:00
Awhite-blue star is hotter than a red star. select the best answer from the choices provided true or false
Answers: 2

You know the right answer?
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus...
Questions

English, 09.01.2020 11:31

Chemistry, 09.01.2020 11:31

Mathematics, 09.01.2020 11:31

Physics, 09.01.2020 11:31



Physics, 09.01.2020 11:31





History, 09.01.2020 11:31



Mathematics, 09.01.2020 11:31

History, 09.01.2020 11:31

History, 09.01.2020 11:31

History, 09.01.2020 11:31

Mathematics, 09.01.2020 11:31

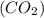 .
.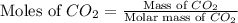
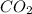 = 44 g/mole
= 44 g/mole