Physics, 12.02.2020 06:02 djfluffyman999
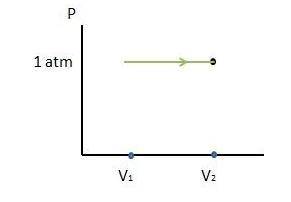
A cylinder contains 0.250 mol of carbon dioxide (CO2) gas at a temperature of 27.0∘C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0∘C. Assume that the CO2 may be treated as an ideal gas. (a) Draw a pV-diagram for this process. (b) How much work is done by the gas in this process? (c) On what is this work done? (d) What is the change in internal energy of the gas? (e) How much heat was supplied to the gas? (f) How much work would have been done if the pressure had been 0.50 atm?

Answers: 3
Another question on Physics

Physics, 21.06.2019 21:30
According to social exchange theory, altruistic behaviors benefit the individual who performs them. t/f
Answers: 1


Physics, 22.06.2019 14:10
Amachinist turns the power on to a grinding wheel, at rest, at time t = 0 s. the wheel accelerates uniformly for 10 s and reaches the operating angular velocity of 96 rad/s. the wheel is run at that angular velocity for 40 s and then power is shut off. the wheel slows down uniformly at 1.5 rad/s2 until the wheel stops. in this situation, the time interval of deceleration is closest to:
Answers: 3

Physics, 22.06.2019 16:20
Specific heat refers to the amount of heat required to change 1 gram of a substance by degree(s) celsius
Answers: 1
You know the right answer?
A cylinder contains 0.250 mol of carbon dioxide (CO2) gas at a temperature of 27.0∘C. The cylinder i...
Questions

History, 20.12.2019 09:31


Chemistry, 20.12.2019 09:31


Mathematics, 20.12.2019 09:31

English, 20.12.2019 09:31


Mathematics, 20.12.2019 09:31


Health, 20.12.2019 09:31





Health, 20.12.2019 09:31





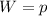 ΔV ------------------------ (i)
ΔV ------------------------ (i)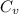 ΔT -------------------- (iv)
ΔT -------------------- (iv)