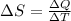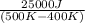Two objects form a closed system. One object, which is at 400 K, absorbs 25.0 kJ of heat from the other object, which is at 500 K. What is the net change in entropy ΔSsysΔSsysDeltaS_sys of the system? Assume that the temperatures of the objects do not change appreciably in the process

Answers: 2
Another question on Physics

Physics, 22.06.2019 00:00
Astate of matter all that has define volume and can flow is a(n)
Answers: 3

Physics, 22.06.2019 04:20
Astone is thrown into a pond. what happens to the amplitude of the resulting waves as they get farther from the point where the stone hit the water? explain.
Answers: 3

Physics, 22.06.2019 06:30
What are similarities and differences between refraction, reflection, diffraction and absorption?
Answers: 3

Physics, 22.06.2019 08:30
A40.0 l tank of ammonia has a pressure of 12.7 kpa. calculate the. volume of the ammonia if it’s pressure is changed to 8.4 kpa while its temperature remains constant.
Answers: 3
You know the right answer?
Two objects form a closed system. One object, which is at 400 K, absorbs 25.0 kJ of heat from the ot...
Questions


Mathematics, 04.01.2020 01:31




Biology, 04.01.2020 01:31



Health, 04.01.2020 01:31



Mathematics, 04.01.2020 01:31

Mathematics, 04.01.2020 01:31


English, 04.01.2020 01:31



English, 04.01.2020 01:31


History, 04.01.2020 01:31

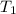 = 400 K,
= 400 K, 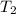 = 500 K,
= 500 K, 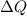 = 25 kJ = 25000 J (as 1 kJ = 1000 J)
= 25 kJ = 25000 J (as 1 kJ = 1000 J)