
Physics, 13.02.2020 23:54 TheOverlordOfWhales
A) Consider 1.3 moles of an ideal gas at an initial temperature of 400 K and in a 1.2 m3 closed container. If the gas goes through an isochoric process to twice the initial temperature, what is the new pressure of the gas in Pa?
b) Consider 1.3 moles of an ideal gas at an initial temperature of 400 K and in a 1.2 m3closed container. If the gas goes through an isothermal process to 3.6 m3, what is the new pressure of the gas in Pa?
c) Consider 1.3 moles of an ideal gas at an initial temperature of 400 K and in a 1.2 m3 closed container. If the gas goes through an isobaric process to 3.6 m3, what is the new temperature of the gas in Kelvin?

Answers: 1
Another question on Physics

Physics, 22.06.2019 01:50
Arod of some material 0.20 m long elongates 0.20 mm on heating from 21 to 120°c. determine the value of the linear coefficient of thermal expansion [in (degrees c)^-1] for this material.
Answers: 2


Physics, 22.06.2019 11:30
You've already seen the value of 9.8 in this lesson. what's this value called? what quantity does it represent?
Answers: 2

Physics, 22.06.2019 23:30
Avector a⃗ has a length of 8.6 m and points in the negative x direction. find the x component of the vector −3.7a⃗ .
Answers: 1
You know the right answer?
A) Consider 1.3 moles of an ideal gas at an initial temperature of 400 K and in a 1.2 m3 closed cont...
Questions

History, 05.04.2021 22:00

Biology, 05.04.2021 22:00

Mathematics, 05.04.2021 22:00


Mathematics, 05.04.2021 22:00

Mathematics, 05.04.2021 22:00

English, 05.04.2021 22:00

Mathematics, 05.04.2021 22:00


Mathematics, 05.04.2021 22:00







Mathematics, 05.04.2021 22:00



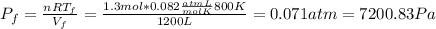
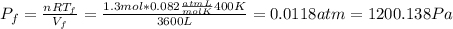
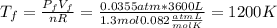
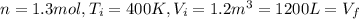
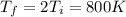 ,
, 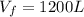 n = 1.3 mol and we can find the final pressure like this:
n = 1.3 mol and we can find the final pressure like this: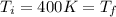 since the process is isothermal
since the process is isothermal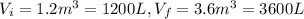
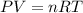
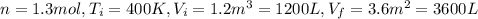
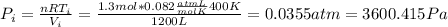
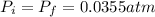 and we can find the final temperature like this:
and we can find the final temperature like this:


