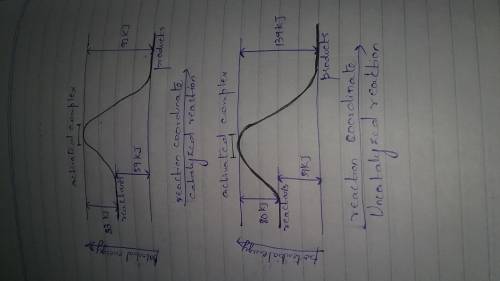
A catalyst decreases the activation energy of a particular exothermic reaction by 47 kJ/mol, to 33 kJ/mol. Assuming that the mechanism has only one step, and that the products are 59 kJ lower in energy than the reactants, sketch approximate energy-level diagrams for the catalyzed and uncatalyzed reactions.

Answers: 2
Another question on Physics

Physics, 21.06.2019 20:40
If an object with an initial temperature of 300 k increases its temperature by 1°c every minute, by how many degrees fahrenheit will its temperature have increased in 10 minutes? (a) 6°f (b) 10°f (c) 18°f (d) 30°f
Answers: 3

Physics, 22.06.2019 09:00
Atelevision set that has been running for a while heats up even the air around it. which two laws of thermodynamics could be used to analyze this scenario
Answers: 1

Physics, 22.06.2019 13:10
Aplane flying horizontally at an altitude of 1 mile and a speed of of 500mih passes directly over a radar station. find the rate at which the distance from the plane to the station is increasing when it is 2mi away from the station.
Answers: 1

Physics, 22.06.2019 19:20
On a hot saturday morning while people are working inside, the air conditioner keeps the temperature inside the building at 22degreesc. at noon the air conditioner is turned off, and the people go home. the temperature outside is a constant 33degreesc for the rest of the afternoon. if the time constant for the building is 4 hr, what will be the temperature inside the building at 4 : 00 font size decreased by 2 upper p . font size decreased by 2 upper m .? at 6 : 00 font size decreased by 2 upper p . font size decreased by 2 upper m .? when will the temperature inside the building reach 24degreesc? 324281
Answers: 3
You know the right answer?
A catalyst decreases the activation energy of a particular exothermic reaction by 47 kJ/mol, to 33 k...
Questions


Mathematics, 07.07.2019 08:00


Biology, 07.07.2019 08:00



Geography, 07.07.2019 08:00

Chemistry, 07.07.2019 08:00


English, 07.07.2019 08:00

History, 07.07.2019 08:00


History, 07.07.2019 08:00

Social Studies, 07.07.2019 08:00



Mathematics, 07.07.2019 08:00


Social Studies, 07.07.2019 08:00

Health, 07.07.2019 08:00