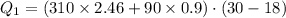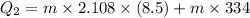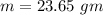Physics, 14.02.2020 19:52 chanavictor2747
Suppose 310. grams of ethanol (ethyl alcohol) is in an aluminum cup of 90.0 grams. Both of these are at 30.0C. A mass m of ice at – 8.5C is taken from a freezer and added to the alcohol in the cup. The final temperature of all the components is 18.0C. Assuming no heat was lost from the system, calculate the mass m of the ice added.

Answers: 2
Another question on Physics

Physics, 21.06.2019 19:30
Agymnast dismounts off the uneven bars in a tuck position with a radius of 0.3m (assume she is a solid sphere) and an angular velocity of 2rev/s. during the dismount she stretches out into the straight position, with a length of 1.5m, (assume she is a uniform rod through the center) for her landing. the gymnast has a mass of 50kg. what is her angular velocity in the straight position?
Answers: 2

Physics, 22.06.2019 14:20
How many atoms of nitrogen are in the chemical formula ni(w on
Answers: 1

Physics, 22.06.2019 18:00
At the negative terminal of the battery the electron has electric potential energy. what happens to this energy as the electron jumps from the negative to the positive terminal?
Answers: 1

Physics, 22.06.2019 21:00
What is the efficiency of an engine that does 80 j of work and exhausts 320 j of heat while taking in 400 j of heat ? a. 10% b. 20% c. 80% d. 25%
Answers: 1
You know the right answer?
Suppose 310. grams of ethanol (ethyl alcohol) is in an aluminum cup of 90.0 grams. Both of these are...
Questions

Mathematics, 27.07.2020 20:01


English, 27.07.2020 20:01


Health, 27.07.2020 20:01

Mathematics, 27.07.2020 20:01

History, 27.07.2020 20:01



English, 27.07.2020 20:01


Engineering, 27.07.2020 20:01



Mathematics, 27.07.2020 20:01

Mathematics, 27.07.2020 20:01

Social Studies, 27.07.2020 20:01

Mathematics, 27.07.2020 20:01


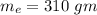
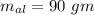
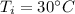
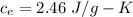
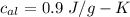
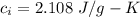
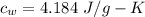
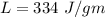
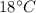 is reached
is reached