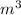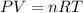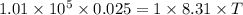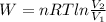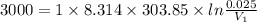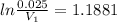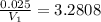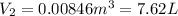Physics, 17.02.2020 21:14 emmmmmily997
One mole of an ideal gas does 3000 J of work on its surroundings as it expands isothermally to a final pressure of 1.00 atm and volume of 25.0 L. Determine:
a) the initial volume ?
b) the temperature of the gas?
(Note: 1 atm = 1.01 x 105Pa, universal gas constant R = 8.31 J/mol K, 1 L = 10-3m3)

Answers: 3
Another question on Physics

Physics, 20.06.2019 18:02
Ajogger runs 5.0 km on a straight trail at and angle of 60 south of west . what is the southern component of the run rounded to the nearest tenth of a kilometer
Answers: 3

Physics, 21.06.2019 23:50
The discovery that the universe appears to be expanding led to a widely accepted theory called a.) the big bang theory b.) the doppler effect c.) hubble’s law d.) solar nebular theory e.) the seyfert theory
Answers: 2

Physics, 22.06.2019 03:00
Which of the following harmful chemicals are found in tobacco smoke? a. carbon monoxide b. carbon dioxide c. nicotine b. carbon dioxide d. both a and c
Answers: 2

Physics, 22.06.2019 12:10
The average density of the planet uranus is 1.27 103 kg/m3. the ratio of the mass of neptune to that of uranus is 1.19. the ratio of the radius of neptune to that of uranus is 0.969. find the average density of neptune.
Answers: 1
You know the right answer?
One mole of an ideal gas does 3000 J of work on its surroundings as it expands isothermally to a fin...
Questions



Computers and Technology, 29.10.2020 16:50





Mathematics, 29.10.2020 16:50



Mathematics, 29.10.2020 16:50





Chemistry, 29.10.2020 16:50

English, 29.10.2020 16:50

English, 29.10.2020 16:50

History, 29.10.2020 16:50

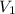 and initial pressure
and initial pressure 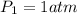 ( As pressure is constant )
( As pressure is constant )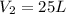 = 0.025
= 0.025