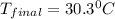An ice cube at 0.00 ∘C with a mass of 21.5 g is placed into 500.0 g of water, initially at 31.0 ∘C, in an insulated container. Part A Assuming that no heat is lost to the surroundings, what is the temperature of the entire water sample after all of the ice has melted?

Answers: 3
Another question on Physics

Physics, 21.06.2019 19:30
Describe the path of a ray that approaches a mirror parallel to the principal axis.
Answers: 2


Physics, 22.06.2019 07:30
Gas cloud 1 is likely to form a star. gas cloud 2 is not. based on this information, match the given conditions with each cloud
Answers: 2

Physics, 22.06.2019 08:00
Based on the concept of the wave-like nature of light, huygens' theory of light postulates that the more light was "bent" by a substance the slower it would move while traversing across that substance. a) deflection b) interference c) refraction d) resonance
Answers: 3
You know the right answer?
An ice cube at 0.00 ∘C with a mass of 21.5 g is placed into 500.0 g of water, initially at 31.0 ∘C,...
Questions

Social Studies, 09.07.2019 17:40


Biology, 09.07.2019 17:40

Mathematics, 09.07.2019 17:40




Biology, 09.07.2019 17:40


History, 09.07.2019 17:40


History, 09.07.2019 17:40


History, 09.07.2019 17:40


Mathematics, 09.07.2019 17:40

Chemistry, 09.07.2019 17:40



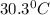
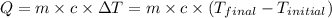 .......(1)
.......(1)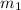 = mass of ice = 21.5 g
= mass of ice = 21.5 g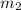 = mass of water = 500.0 g
= mass of water = 500.0 g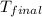 = final temperature = ?
= final temperature = ?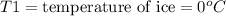
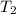 = temperature of water =
= temperature of water =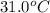
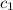 = specific heat of ice=
= specific heat of ice= 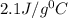
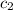 = specific heat of water =
= specific heat of water = 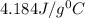
![21.5\times 2.1\times (T_{final}-0)=-[500.0\times 4.184\times (T_{final}-31.0)]](/tpl/images/0513/4270/003f3.png)