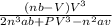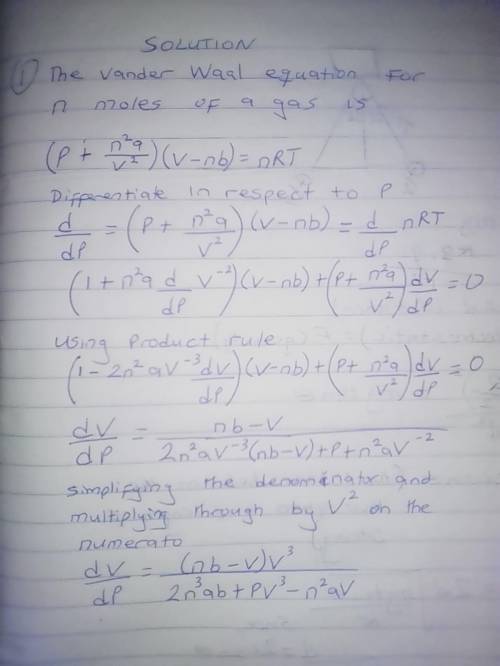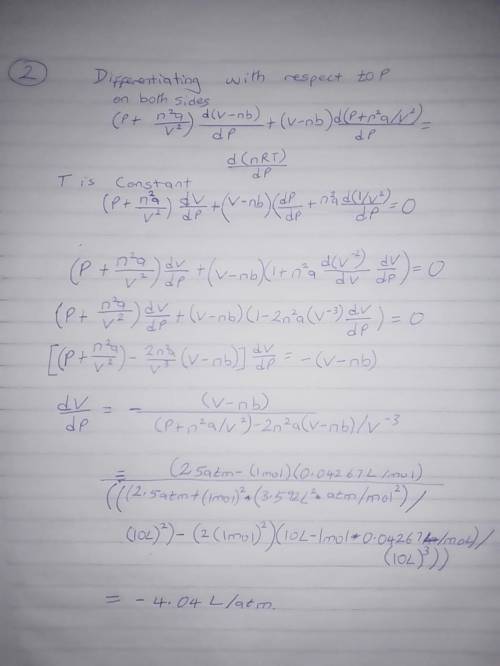Physics, 20.02.2020 16:46 izzysmith6836
The van der Waals equation for n moles of a gas is where P is the pressure, V is the volume, and T is the temperature of the gas. The constant R is the universal gas constant and a and b are positive constants that are characteristic of a particular gas. a) If T remains constant, use implicit differentiation to find . b) Find the rate of change of volume with respect to pressure of 1 mole of carbon dioxide at a volume of V = 10 L and a pressure of P = 2.5 atm. Use a = 3.592 L2 -atm/mole2 and b = 0.04267 L/mole.

Answers: 3
Another question on Physics

Physics, 21.06.2019 15:10
Consider the following problem: a box with an open top is to be constructed from a square piece of cardboard, 3 ft wide, by cutting out a square from each of the four corners and bending up the sides. find the largest volume that such a box can have.
Answers: 3

Physics, 22.06.2019 09:30
Which of these is not a possible type of energy transformation? a. electrical energy into light energy b. sound energy into nuclear energy c. potential energy into kinetic energy d. kinetic energy into mechanical energy
Answers: 1

Physics, 22.06.2019 14:40
An athlete is holding 24 lb of weights at a height of 6 inches above the stack as shown. to lower the weights, she applies a constant force of 5 lb to the handle. determine the velocity of the weights immediately before they hit the stack.
Answers: 1

Physics, 23.06.2019 03:30
As you lift an 88 n box straight upward you produce a power of 72 w. what is the speed of the box?
Answers: 2
You know the right answer?
The van der Waals equation for n moles of a gas is where P is the pressure, V is the volume, and T i...
Questions

Mathematics, 27.06.2019 21:30










Business, 27.06.2019 21:30


Mathematics, 27.06.2019 21:30

History, 27.06.2019 21:30

Mathematics, 27.06.2019 21:30

History, 27.06.2019 21:30


Computers and Technology, 27.06.2019 21:30

Spanish, 27.06.2019 21:30