Physics, 21.02.2020 03:34 maskythegamer
Helium gas is in a cylinder that has rigid walls. If the pressure of the gas is 2.00 atm, then the root-mean-square speed of the helium atoms is vrms = 176 m/s. By how much (in atmospheres) must the pressure be increased to increase the vrms of the He atoms by 100 m/s? Ignore any change in the volume of the cylinder

Answers: 2
Another question on Physics

Physics, 22.06.2019 04:20
Awave is produced in a rope. the wave has a speed of 33 m/s and a frequency of 22 hz. what wavelength is produced?
Answers: 2

Physics, 22.06.2019 15:10
When electrons are added to the outermost shell of a carbon atom, it forms--an anion that has a larger anion that has a smaller cation that has a larger cation that has a smaller radius.
Answers: 3

Physics, 22.06.2019 15:50
If the work required to stretch a spring 3 ft beyond its natural length is 15 ft-lb, how much work is needed to stretch it 27 in. beyond its natural length?
Answers: 1

Physics, 22.06.2019 16:30
3. a lunar exploration vehicle was created by a research team. it weighs 3,000 kg on the earth. it needs an acceleration of 10 m/s2 on the moon. in order to have the same acceleration, what will be the net force acting on the vehicle on the earth?
Answers: 2
You know the right answer?
Helium gas is in a cylinder that has rigid walls. If the pressure of the gas is 2.00 atm, then the r...
Questions


Mathematics, 14.07.2019 16:00

Mathematics, 14.07.2019 16:00

History, 14.07.2019 16:00

Mathematics, 14.07.2019 16:00


Health, 14.07.2019 16:00




Biology, 14.07.2019 16:00

Mathematics, 14.07.2019 16:00

Computers and Technology, 14.07.2019 16:00


History, 14.07.2019 16:00




Physics, 14.07.2019 16:00

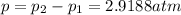
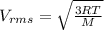
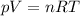
![\frac{V_{rms1} }{V_{rms2}}=\frac{\sqrt{p_{1} } }{\sqrt{p_{2}} }\\ p_{2}=[\frac{(V_{rms1})^{2} }{(V_{rms2})^{2} }]p_{1}\\ p_{2}=\frac{(276m/s)^{2} }{(176m/s)^{2} } (2atm)\\p_{2}=4.9188atm](/tpl/images/0518/7233/c9a71.png)