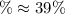Physics, 21.02.2020 23:05 ciralove2004
Consider the elementary gas-phase reversible reaction A 3C Pure A enters at a temperature of 400 K and a pressure of 10 atm. At this temperature, KC 0.25(mol/dm3)2. Calculate the equilibrium conversion for each of the following situations:

Answers: 1
Another question on Physics

Physics, 22.06.2019 00:10
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 05:30
What is a neurotransmitter involved in mood reward, addiction, and motor behaviors?
Answers: 3

Physics, 22.06.2019 19:30
Another word for electromagnetic energy is a. heat b. force c. matter d. radiation
Answers: 1

Physics, 23.06.2019 01:00
First answer marked as brainiest which is the correct equation describing the first law of thermodynamics? a) when the internal energy of a system changes, it equals the amount of heat added plus the work done. b) when work is done by the piston, it is equal to the heat added plus the change in internal energy of the system. c) when heat is added to the system, it is equal to the amount of work done by the piston plus the change in internal energy of the system.
Answers: 2
You know the right answer?
Consider the elementary gas-phase reversible reaction A 3C Pure A enters at a temperature of 400 K a...
Questions


Geography, 27.06.2019 08:00



Mathematics, 27.06.2019 08:00


Biology, 27.06.2019 08:00

Mathematics, 27.06.2019 08:00





Geography, 27.06.2019 08:00




Mathematics, 27.06.2019 08:00



Mathematics, 27.06.2019 08:00



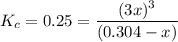

![\% = [0.304mol/liter-0.1845mol/liter]/(0.304mol/liter)\times 100](/tpl/images/0519/7722/78e3b.png)