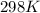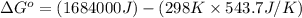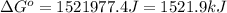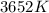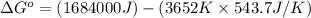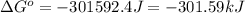The chemical reaction that causes iron to corrode in air is given by
4Fe + 3O2→2Fe2O3 in which...

Physics, 21.02.2020 23:38 ellycleland16
The chemical reaction that causes iron to corrode in air is given by
4Fe + 3O2→2Fe2O3 in which at 298 K
ΔHrxn= 1684 kJ
ΔSrxn= 543.7 J/K
Part A. What is the standard Gibbs free energy for this reaction? Assume the commonly used standard reference temperature of 298 K.
Part B. What is the Gibbs free energy for this reaction at 3652 K? Assume that Delta H and Delta S do not change with temperature.

Answers: 1
Another question on Physics

Physics, 21.06.2019 20:00
What happens to atoms and chemical bonds during a reaction?
Answers: 1

Physics, 22.06.2019 00:30
What are the theoretical properties of a gas at a temperature of 0 kelvin?
Answers: 3

Physics, 22.06.2019 15:30
What is a subatomic particle with a negative charge and very little mass?
Answers: 1

Physics, 22.06.2019 16:00
Frank just graduated from eighth grade. assuming exactly four years from now he will graduate from high school how many seconds does he have until his high school graduation
Answers: 2
You know the right answer?
Questions



Mathematics, 28.01.2020 17:48

English, 28.01.2020 17:48


English, 28.01.2020 17:48


Mathematics, 28.01.2020 17:48

History, 28.01.2020 17:48


History, 28.01.2020 17:48

Mathematics, 28.01.2020 17:48

Social Studies, 28.01.2020 17:48

Mathematics, 28.01.2020 17:48




Business, 28.01.2020 17:48

Computers and Technology, 28.01.2020 17:49

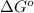 at 298 K is, 1521.9 kJ
at 298 K is, 1521.9 kJ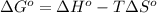
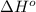 = standard enthalpy = 1684 kJ = 1684000 J
= standard enthalpy = 1684 kJ = 1684000 J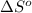 = standard entropy = 543.7 J/K
= standard entropy = 543.7 J/K