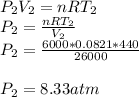Physics, 22.02.2020 02:49 nathanbrockdac
A sealed 26-m3 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 440 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol·K = 0.0821 L·atm/mol·K. The final pressure of the gas is closest to
A) 0.31
B) 0.34
C) 0.33
D) 0.36
E) 0.29

Answers: 2
Another question on Physics

Physics, 21.06.2019 23:20
The 5-kg cylinder is initially at rest when it is placed in contact with the wall b and the rotor at a. if the rotor always maintains a constant clockwise angular velocity v = 6 rad> s, determine the initial angular acceleration of the cylinder. the coefficient of kinetic friction at the contacting surfaces b and c is mk = 0.2.
Answers: 3

Physics, 22.06.2019 12:30
Brades exam guidelines exam instructions billing & payments & support question 6 of 20 : select the best answer for the question use this illustration to answer the question below. forms & resources al programs 9 ww linics 6. in the circuit shown in the figure above, suppose that the value of r, is 100k, and the value of r2 is 470 kq. at which of the following locations in the circuit would you measure the highest voltage with your meter? rvices 999999 a. between points b and c b. between points a and b c. between points b and e d. between points a and c eredu mark for review (will be highlighted on the review page) mend < < previous question next question
Answers: 1


You know the right answer?
A sealed 26-m3 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K....
Questions


Mathematics, 10.07.2019 04:30





Mathematics, 10.07.2019 04:30






Mathematics, 10.07.2019 04:30

Mathematics, 10.07.2019 04:30






Mathematics, 10.07.2019 04:30