
Mercury has a density of 13.6 g/cm , whereas the density of water is 1.00 g/cm. The pressure of the atmosphere is high enough to push mercury 76.0 cm up
through a vacuum tube. How high can a column of water be pushed up in a vacuum tube by the atmosphere? (Hint: The level of either liquid rises till the atmospheric
pressure is in balance with the pressure by the liquid computed by density xgx height; since the atmospheric pressure is the same in both scenarios, one only
needs to compare the liquid pressures to find the height of water) (Express the height of water in meters and with 3 significant figures in the form XX. X; number only
- no units)

Answers: 2
Another question on Physics

Physics, 22.06.2019 10:00
Because air contracts as it cools, the air pressure inside a freezer is typically lower than on the outside. why do ice cubes inside a freezer tend to shrink over time? a. the ice dissolves oxygen from the air, forming a denser crystalline matrix.b. the ice reacts chemically with carbon dioxide in the air, forming gaseous carbon compounds.c. the ice melts, and then the liquid freezes as ice crystals on the bottom of the freezer.d. the ice sublimes, and then the water vapor deposits as ice crystals on the sides of the freezer.
Answers: 1

Physics, 22.06.2019 16:00
At what time will the interfering waves have an amplitudeof zero?
Answers: 1

Physics, 22.06.2019 16:30
The air in an automobile tire with a volume of 2.60 ft3 is at 70°f and 21 psig. determine the amount of air that must be added to raise the pressure to the recommended value of 30 psig. assume the atmospheric pressure to be 14.6 psia and the temperature and the volume to remain constant. the gas constant of air is ru = 53.34ft⋅lbflbm⋅r(1 psia144 lbf/ft2) = 0.3704 psia⋅ft3lbm⋅r the amount of air that must be added to raise the pressure is lbm
Answers: 3

Physics, 22.06.2019 19:10
Global warming will produce rising sea levels partly due to melting ice caps but also due to the expansion of water as average ocean temperatures rise. to get some idea of the size of this effect, calculate the change in length (in m) of a column of water 1.45 km high for a temperature increase of 1.12°c. assume the column is not free to expand sideways. as a model of the ocean, that is a reasonable approximation, as only parts of the ocean very close to
Answers: 3
You know the right answer?
Mercury has a density of 13.6 g/cm , whereas the density of water is 1.00 g/cm. The pressure of the...
Questions

Mathematics, 03.07.2020 14:01

Mathematics, 03.07.2020 14:01

Health, 03.07.2020 14:01

Mathematics, 03.07.2020 14:01

Health, 03.07.2020 14:01

English, 03.07.2020 14:01


Mathematics, 03.07.2020 14:01

Chemistry, 03.07.2020 14:01




Mathematics, 03.07.2020 14:01


Social Studies, 03.07.2020 14:01

Mathematics, 03.07.2020 14:01




Mathematics, 03.07.2020 14:01

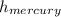 *
* 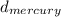 * g =
* g = 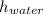 *
* 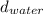 * g
* g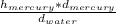 = (13.6 g/cm * 76 cm)/1 g/cm = 1033.6 cm
= (13.6 g/cm * 76 cm)/1 g/cm = 1033.6 cm

