
Calculate the energy in the form of heat (in kJ) required to change 75.0 g of liquid water at 27.0 °C to ice at –20.0 °C. Assume that no energy in the form of heat is transferred to the environment. (Heat of fusion = 333 J/g; heat of vaporization = 2256 J/g; specific heat capacities: ice = 2.06 J/g·K, liquid water = 4.184 J/g·K)

Answers: 3
Another question on Physics

Physics, 21.06.2019 21:00
Mike walks 100 meter north, then walks 30 meters south. after this, he walks another 10 meters north. what is the magnitude of his total displacement during this walk,in meters?
Answers: 2


Physics, 22.06.2019 15:20
What component of earth’s atmosphere exists entirely as a result of photosynthesis?
Answers: 2

Physics, 22.06.2019 19:00
The friction of the water on a boat produces an acceleration of -10. m/s2. if the boat is traveling at 30. m/s and the motor is shut off, how long it take the boat to slow down to 5.0 m/s?
Answers: 3
You know the right answer?
Calculate the energy in the form of heat (in kJ) required to change 75.0 g of liquid water at 27.0 °...
Questions

Mathematics, 01.09.2021 07:10

Computers and Technology, 01.09.2021 07:10

Business, 01.09.2021 07:10


Mathematics, 01.09.2021 07:10


Biology, 01.09.2021 07:10

Geography, 01.09.2021 07:10



Mathematics, 01.09.2021 07:10


English, 01.09.2021 07:10

English, 01.09.2021 07:10



Mathematics, 01.09.2021 07:10


Mathematics, 01.09.2021 07:10

Mathematics, 01.09.2021 07:20

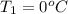
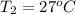
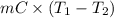
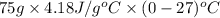
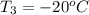 = (20 + 273) K = 293 K and specific heat of ice is 2.108 kJ/kg K.
= (20 + 273) K = 293 K and specific heat of ice is 2.108 kJ/kg K. 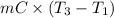
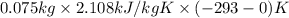
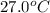 to ice at
to ice at 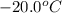 is -37.86 kJ.
is -37.86 kJ.

