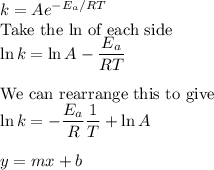Physics, 25.02.2020 23:32 deanazilyiah
Consider the reaction data. A ⟶ products T ( K ) k ( s − 1 ) 225 0.385 525 0.635 What two points should be plotted to graphically determine the activation energy of this reaction? To avoid rounding errors, use at least three significant figures in all values. x 1 = y 1 = x 2 = y 2 = Determine the rise, run, and slope of the line formed by these points. rise: run: slope: What is the activation energy of this reaction? E a = J / mol

Answers: 1
Another question on Physics


Physics, 22.06.2019 22:00
Four friends, a, b, c, and d are standing varying distances away from a speaker which is producing sound waves.
Answers: 2

Physics, 23.06.2019 00:00
Which is an advantage of subdividing science into different areas?
Answers: 3

Physics, 23.06.2019 01:40
When you see distant streetlights through smog, they look dimmer and redder than they do normally. but when you see the same streetlights through fog or falling snow, they look dimmer but not redder. use your knowledge of the interstellar medium to discuss the relative sizes of the particles in smog, fog, and snowstorms compared to the wavelength of light.
Answers: 3
You know the right answer?
Consider the reaction data. A ⟶ products T ( K ) k ( s − 1 ) 225 0.385 525 0.635 What two points sho...
Questions



Spanish, 06.02.2022 09:20

Geography, 06.02.2022 09:20



Computers and Technology, 06.02.2022 09:20


Mathematics, 06.02.2022 09:20



Mathematics, 06.02.2022 09:20


Computers and Technology, 06.02.2022 09:20

Mathematics, 06.02.2022 09:20

Mathematics, 06.02.2022 09:20

Chemistry, 06.02.2022 09:20

Business, 06.02.2022 09:20


Biology, 06.02.2022 09:20