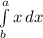Physics, 26.02.2020 00:58 jessezarate4513
Carbon dioxide (CO2) gas in a piston-cylinder assembly undergoes three processes in series that begin and end at the same state (a cycle).
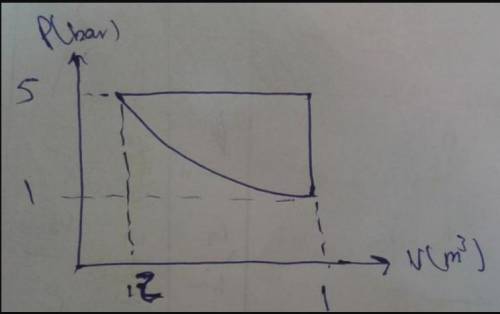
Process 1-2: Expansion from State 1 where p1 = 10 bar, V1 = 1 m3 , to State 2 where V2 = 4 m3 . During the process, pressure and volume are related by pV1.5 = constant.
Process 2-3: Constant volume heating to State 3 where p3 = 10 bar.
Process 3-1: Constant pressure compression to State
Sketch the processes on p-V coordinates and evaluate the work for each process, in kJ. What is net work for the cycle, in kJ?

Answers: 1
Another question on Physics

Physics, 22.06.2019 09:30
How would a small bar magnet be oriented when placed at position x?
Answers: 2

Physics, 23.06.2019 17:00
A10.0 kg crate is pushed with a horizontal force of 40 n. the crate moves at a constant velocity of 3.0 m/s. what is the value of the friction force on the crate? 10 n 100 n 40 n 70 n
Answers: 1

Physics, 24.06.2019 01:00
Calculate the equilibrium number of vacancies per cubic meter for copper at 27°c and 1000°c, respectively. assume that the energy for vacancy formation is 0.9 ev/atom; the atomic weight and density (at both 27 and 1000 ° c) for copper are 63.5 g/mol and 8.4 g/cm3, respectively
Answers: 1

Physics, 24.06.2019 01:00
Aball is thrown with a speed of 10 m/s at an angle of 65. which of the following statement describes the motion of the ball at the top of its trajectorya. the horizontal component of velocity is increasing b. the horizontal component of velocity is zeroc. the vertical acceleration is zerod. the vertical acceleration is constant
Answers: 1
You know the right answer?
Carbon dioxide (CO2) gas in a piston-cylinder assembly undergoes three processes in series that begi...
Questions



Mathematics, 03.12.2020 06:10

Mathematics, 03.12.2020 06:10

Arts, 03.12.2020 06:10

Mathematics, 03.12.2020 06:10

Biology, 03.12.2020 06:10


SAT, 03.12.2020 06:10

Mathematics, 03.12.2020 06:10

Mathematics, 03.12.2020 06:10

Mathematics, 03.12.2020 06:10

English, 03.12.2020 06:10


Arts, 03.12.2020 06:10



History, 03.12.2020 06:10

Mathematics, 03.12.2020 06:10