Physics, 26.02.2020 03:54 1341220857
A sample of quartz, which has a specific heat capacity of 0.730 Jg1, is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The quartz sample starts off a insulated 95.8 °C and the temperature of the water starts off at 15.0 °C, when the temperature of the water stops changing it's 17.7 °C. The pressure remains constant at 1 atm.
Calculate the mass of the quartz sample.

Answers: 3
Another question on Physics

Physics, 22.06.2019 04:30
Light from the sun reaches the earth in 8.3 minutes. the velocity is 3.00 x 10^8 m/s. how far is earth from the sun? i know how to get to d = (3 x 10^8 m/s)(498 sec). i just don't know how to get from there to the answer being 1.5 x 10^11 m.
Answers: 3

Physics, 22.06.2019 14:40
What is the orbital period of a spacecraft in a low orbit near the surface of mars? the radius of mars is 3.4×106m.
Answers: 2

Physics, 22.06.2019 18:10
Which of the following statement is false ? a.batteries should never be stored on metal trays or outside in the elements)b, battery acid cannot be neutralized)c.do not stack batteries on top of each other)d.never put a damaged battery in a dumpster. i
Answers: 1

Physics, 23.06.2019 01:30
If the pressure in a gas is doubled while its volume is held constant. true or false
Answers: 3
You know the right answer?
A sample of quartz, which has a specific heat capacity of 0.730 Jg1, is put into a calorimeter (see...
Questions

Mathematics, 09.04.2021 18:20


Mathematics, 09.04.2021 18:20

Mathematics, 09.04.2021 18:20



Arts, 09.04.2021 18:20

Mathematics, 09.04.2021 18:20





Mathematics, 09.04.2021 18:20


Mathematics, 09.04.2021 18:20


English, 09.04.2021 18:20

History, 09.04.2021 18:20

French, 09.04.2021 18:20

Mathematics, 09.04.2021 18:20

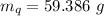
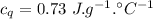
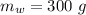
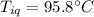
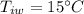
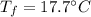
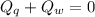
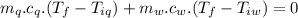
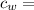 specific heat of water =
specific heat of water = 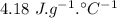 ,
,